ASSURING VACCINE SAFETY Basic Course for a Complex

ASSURING VACCINE SAFETY: Basic Course for a Complex Issue • WELCOME & INTRODUCTIONS • CLINICAL IMMUNIZATION SAFETY ASSESSMENT CENTERS (CISA) • VACCINE ADVERSE EVENT REPORTING SYSTEM (VAERS) • VACCINE SAFETY STUDIES & VACCINE SAFETY DATALINK (VSD) • MISCONCEPTIONS & RISK COMMUNICATION • QUESTIONS & ANSWERS

OBJECTIVES • Discuss 2 functions of government agencies to assure vaccine safety • Identify 2 vaccine safety responsibilities of the clinician • Discuss how vaccines are monitored for safety after they are licensed • List 2 vaccine safety resources

Historical Perspective
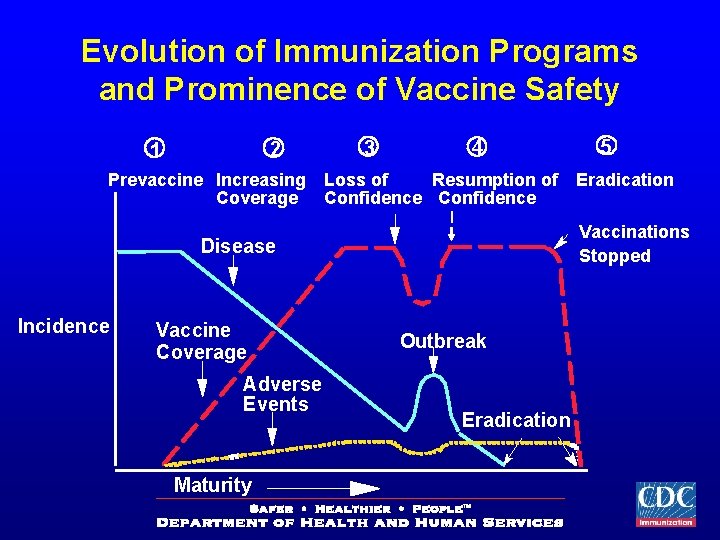
Evolution of Immunization Programs and Prominence of Vaccine Safety 2 1 Prevaccine Increasing Coverage 3 4 Loss of Resumption of Confidence Vaccine Coverage Adverse Events Maturity Eradication Vaccinations Stopped Disease Incidence 5 Outbreak Eradication
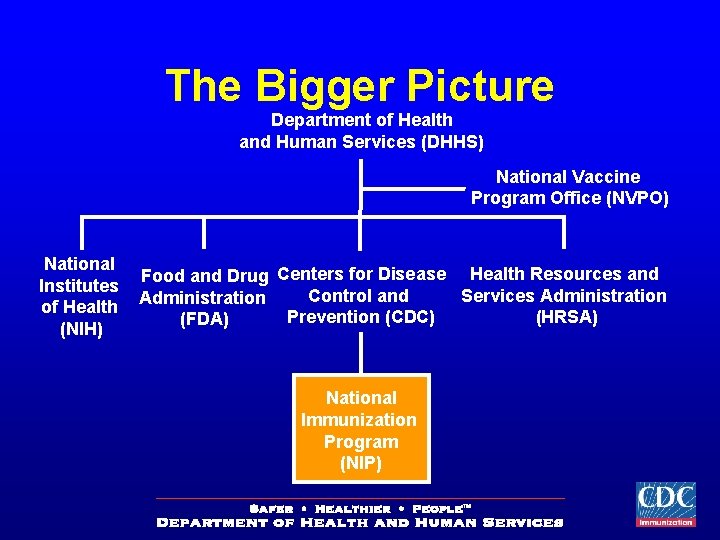
The Bigger Picture Department of Health and Human Services (DHHS) National Vaccine Program Office (NVPO) National Institutes of Health (NIH) Food and Drug Centers for Disease Health Resources and Control and Services Administration Prevention (CDC) (HRSA) (FDA) National Immunization Program (NIP)

Two Licenses Required • The FDA gives a biologic license for the vaccine itself • A separate license is also needed for the manufacturing plant where the vaccine is made.
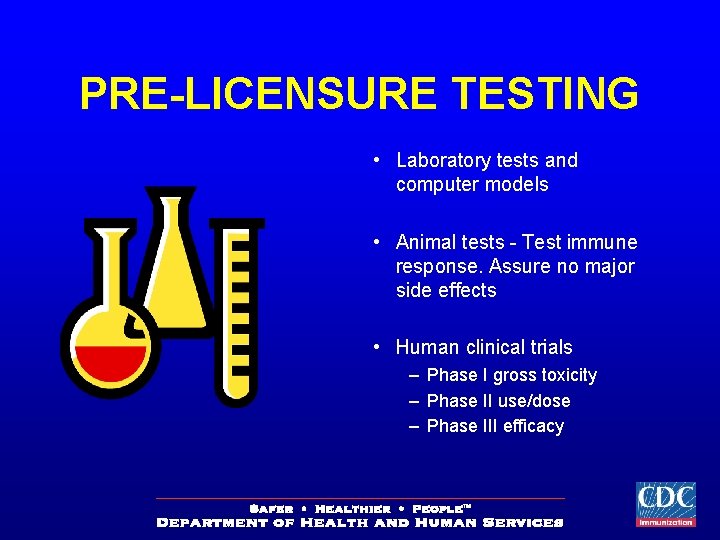
PRE-LICENSURE TESTING • Laboratory tests and computer models • Animal tests - Test immune response. Assure no major side effects • Human clinical trials – Phase I gross toxicity – Phase II use/dose – Phase III efficacy

Every Lot Of Vaccine is Tested and Sampled • After approval, samples of each lot of a vaccine must be submitted to the FDA before it can be released for use. • Tested for safety, potency, and purity

ROLE OF THE CLINICIAN IN VACCINE SAFETY • Proper storage and administration • Identify contraindications • Education • Report and treat Reactions • Refer as appropriate • Follow up
VACCINE MANAGEMENT • Storage and Handling • Timing and Spacing • Administration Issues – Equipment – Injection site recommendations

CDC VACCINE INFORMATION STATEMENTS • Public Health law requires them to be provided to parents at each visit • Contains Vaccine Safety information • Referral information

SPECIAL POPULATIONS • People with moderate or severe illness • History of serious vaccine reaction • Pregnant women • Recent Blood Product Recipients

WHEN IS IT SAFE TO IMMUNIZE? • • Mild illness Disease exposure Antibiotic therapy Breast Feeding Premature birth Most allergies Family history of vaccine reaction

VACCINE REACTIONS • Local Reactions • Systemic Reactions • Allergic Reactions • Emotional

VACCINE REACTIONS What You Can Do • React to needs – Physical – Emotional • Refer – Specialists – Information • Reassure • Report • Follow up

GOVERNMENT’s ROLE • federal agencies involved in vaccine safety: – FDA – CDC – HRSA • Activities include: – – Licensure/Regulations Recommendations Surveillance/Research Injury Compensation
POST-LICENSURE MONITORING • Clinical Immunization Safety Assessment (CISA) Network • Vaccine Adverse Events Reporting System (VAERS) • Vaccine Safety Datalink (VSD) Project
CLINICAL IMMUNIZATION SAFETY ASSESSMENT (CISA) Network • Designed to improve scientific understanding of vaccine safety issues at the individual “patient” level. • Activities – – Evaluate known reactions Evaluate new and unusual events Provide consultation to physicians Develop clinical protocols

CISA Centers • Johns Hopkins (MD) • University of Maryland (MD) • Northern California Kaiser Permanente (CA) • Stanford University (CA) • Vanderbilt University (TN) • Boston University Medical Center (MA) • Columbia-New York Presbyterian Hospital (NYC)

1 -800 -822 -7967 www. vaers. org • Unified national spontaneous reporting system/passive surveillance • Jointly administered by CDC and FDA since 1990 • Receives ~14, 000 reports per year • “Registry” of adverse events

TYPES OF ADVERSE EVENTS REPORTED TO VAERS · Vaccine reaction or side effect · Vaccine potentiated · Programmatic or human error · Coincidental
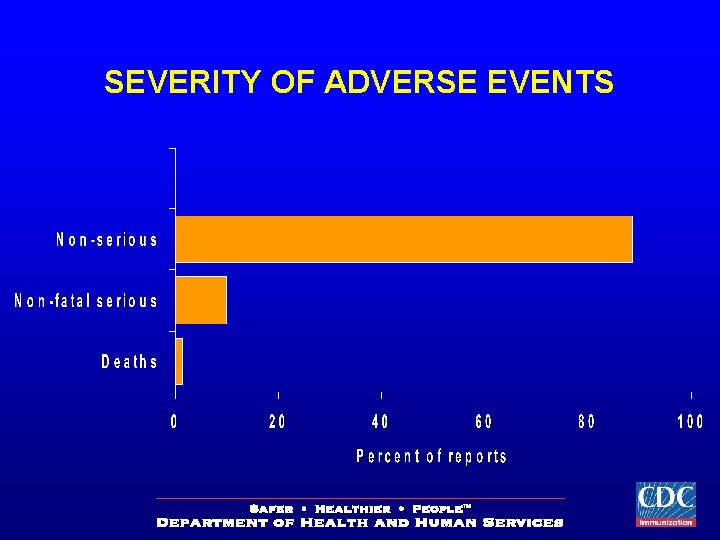
SEVERITY OF ADVERSE EVENTS
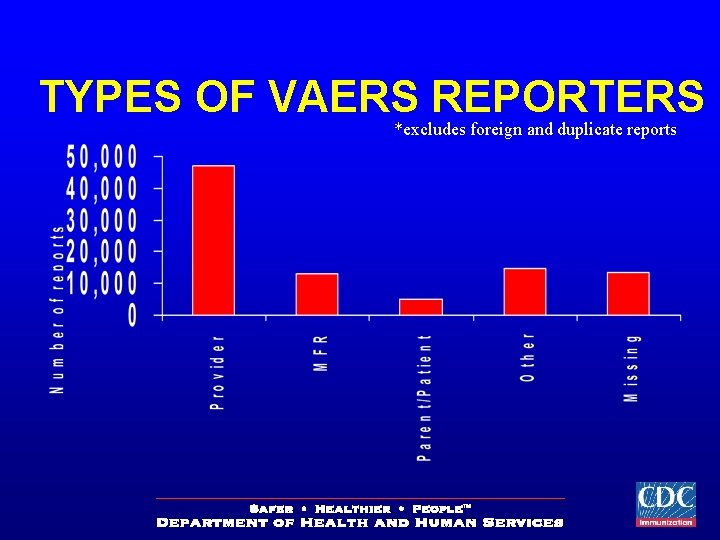
TYPES OF VAERS REPORTERS *excludes foreign and duplicate reports
CASE FOLLOW UP • Letter to Reporters • Serious cases – Medical records • Deaths – Autopsy reports • Not recovered – 60 day follow up – 1 year follow up
What is done with VAERS data? • Detects potential new vaccine side effects not previously known • Detects potential changes in rate of known side effects • Detects potential risk factors for adverse events

VAERS STRENGTHS • National scope • Detects very rare events • Timely reporting • Generates signals • Low cost

VAERS WEAKNESSES • • Complexity Coincidental events Statistical limitations Can not determine vaccine causality by case • Additional studies required to confirm
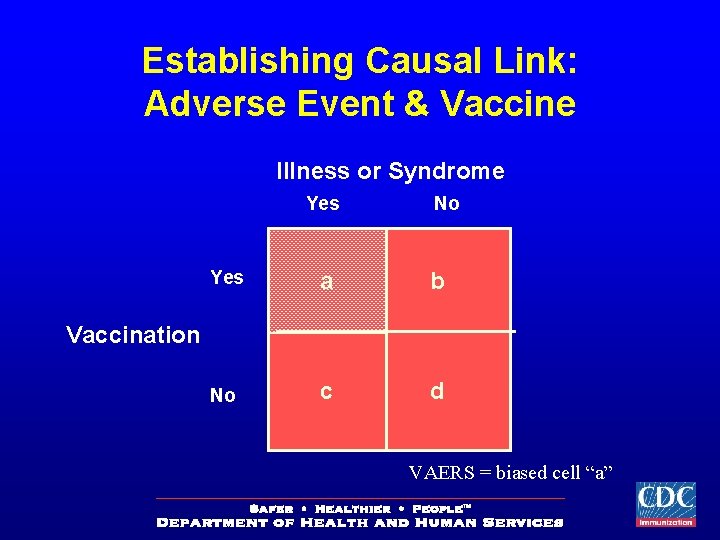
Establishing Causal Link: Adverse Event & Vaccine Illness or Syndrome Yes No Yes a b No c d Vaccination VAERS = biased cell “a”
KEY FACTORS TO ESTABLISH CAUSALITY • Illness always follows exposure • Dose-response • Biologic plausibility • Unique symptoms/lab • Seen in populations greater than chance • Able to be replicated • Alternatives explained

Temporal association does not prove causation.

Vaccine Adverse Event does not mean the vaccine CAUSED the event
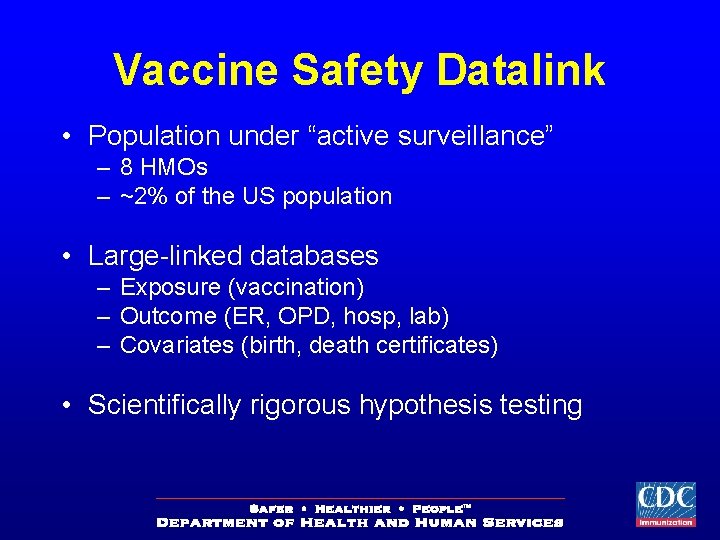
Vaccine Safety Datalink • Population under “active surveillance” – 8 HMOs – ~2% of the US population • Large-linked databases – Exposure (vaccination) – Outcome (ER, OPD, hosp, lab) – Covariates (birth, death certificates) • Scientifically rigorous hypothesis testing
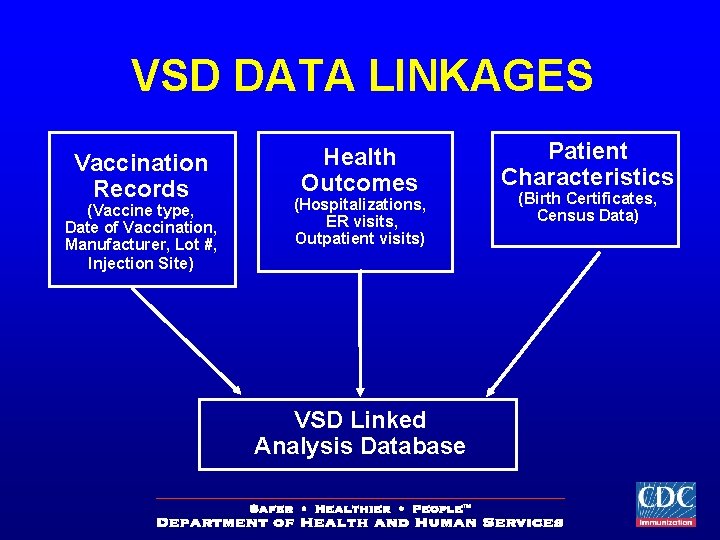
VSD DATA LINKAGES Vaccination Records (Vaccine type, Date of Vaccination, Manufacturer, Lot #, Injection Site) Health Outcomes (Hospitalizations, ER visits, Outpatient visits) VSD Linked Analysis Database Patient Characteristics (Birth Certificates, Census Data)

VSD SELECTED FINDINGS • Diabetes: did not find a risk after Hib or HBV vaccine • Multiple Sclerosis or Optic Neuritis: did not detect a risk after HBV vaccine • Intussusception: noted increased risk in Days 3 – 7 after rotavirus vaccine • Seizures: detected an association with vaccination (MMR and DTP) and febrile seizure but no association with long term seizure disorders

Hot Topics in Vaccine Safety

AUTISM AND VACCINES • Theory posed that MMR vaccine might play a role in autism • Weight of scientific evidence does not support

Studies: Autism and Vaccines • VSD: – Thimerosal and Autism Study • CDC: – MMR/Autism Biopsy Study – NIH MMR/Regression Autism Study – Autism and Thimerosal-Containing Vaccines: Lack of Consistent Evidence for an Association – MMR and Autism Population-based Study in Denmark – Metropolitan Atlanta Developmental Disabilities Surveillance Program

Mercury And Vaccines • Federal Act to reduce mercury exposure • Thimerosal -mercury based preservative • Vaccine schedule prior to 1999 for some infants could exceed 1 federal mercury guideline • No evidence of harm • US vaccines now virtually mercury-free
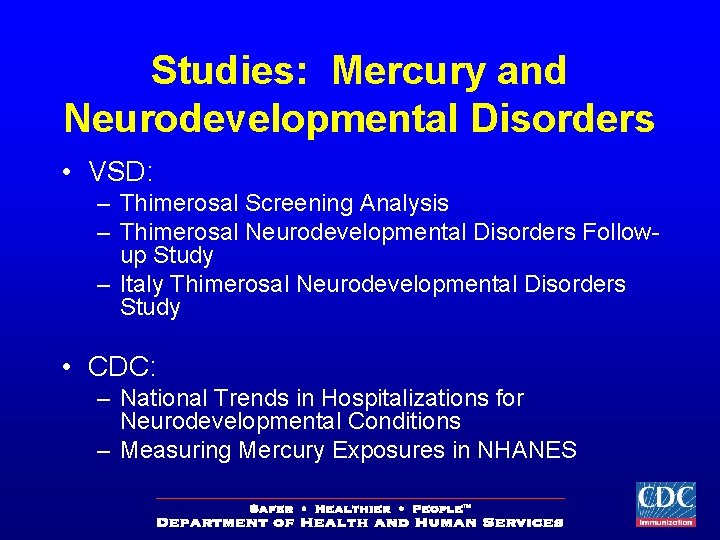
Studies: Mercury and Neurodevelopmental Disorders • VSD: – Thimerosal Screening Analysis – Thimerosal Neurodevelopmental Disorders Followup Study – Italy Thimerosal Neurodevelopmental Disorders Study • CDC: – National Trends in Hospitalizations for Neurodevelopmental Conditions – Measuring Mercury Exposures in NHANES

Vaccine Risk Communication • Dynamic two way process • Informed decision making • Discuss risk uncertainties
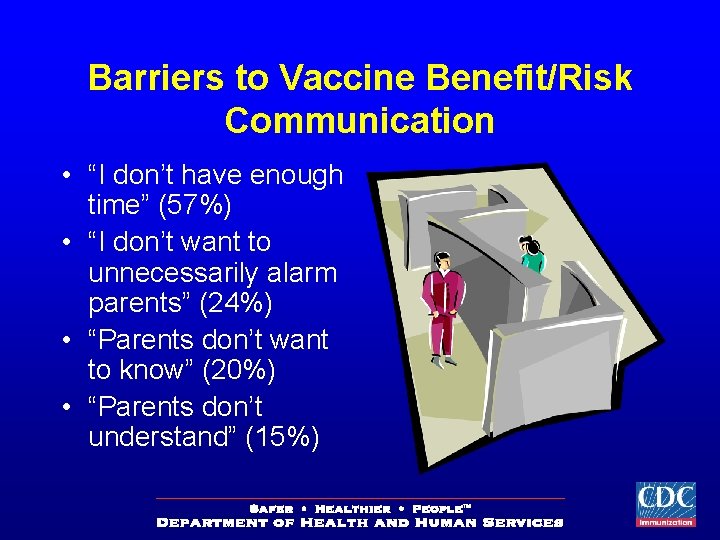
Barriers to Vaccine Benefit/Risk Communication • “I don’t have enough time” (57%) • “I don’t want to unnecessarily alarm parents” (24%) • “Parents don’t want to know” (20%) • “Parents don’t understand” (15%)
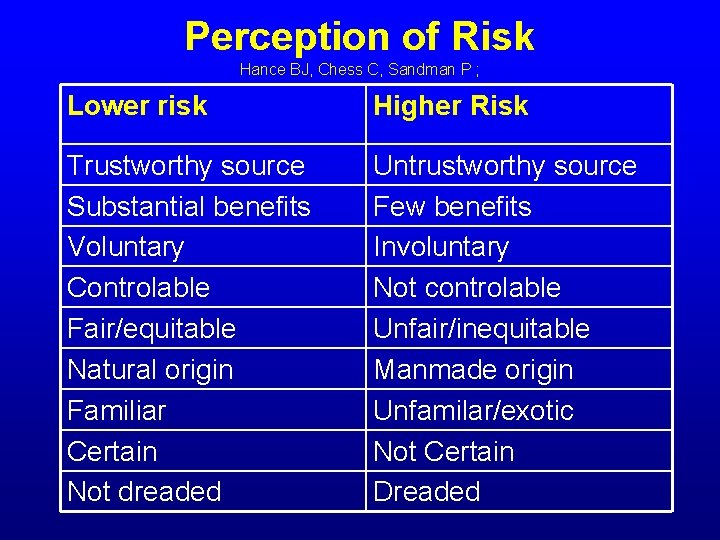
Perception of Risk Hance BJ, Chess C, Sandman P ; Lower risk Higher Risk Trustworthy source Substantial benefits Voluntary Controlable Fair/equitable Natural origin Familiar Certain Not dreaded Untrustworthy source Few benefits Involuntary Not controlable Unfair/inequitable Manmade origin Unfamilar/exotic Not Certain Dreaded
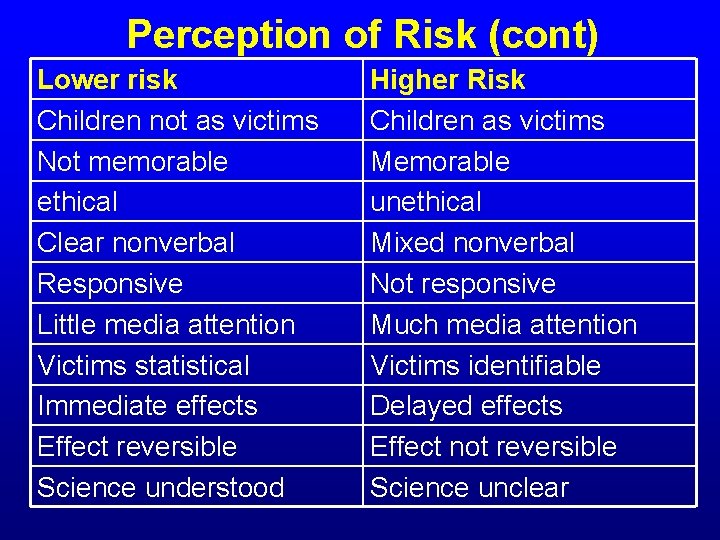
Perception of Risk (cont) Lower risk Children not as victims Not memorable ethical Clear nonverbal Responsive Little media attention Victims statistical Immediate effects Effect reversible Science understood Higher Risk Children as victims Memorable unethical Mixed nonverbal Not responsive Much media attention Victims identifiable Delayed effects Effect not reversible Science unclear

When to Use Risk Communication Vincent T. Covello, Ph. D. Center for Risk Communication High Concern Low Trust (essential) High Concern High Trust (essential) Low Concern Low Trust (recommended) Low Concern High Trust (optional)
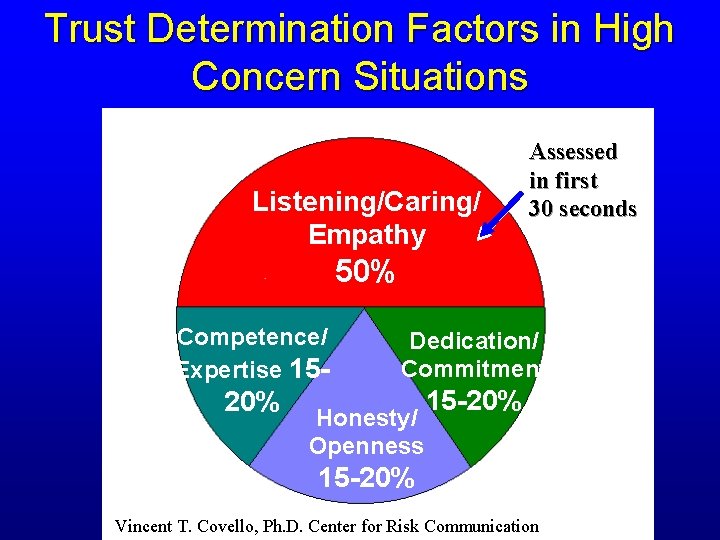
Trust Determination Factors in High Concern Situations Listening/Caring/ Empathy Assessed in first 30 seconds 50% Competence/ Expertise 15 - Dedication/ Commitment 20% 15 -20% Honesty/ Openness 15 -20% Vincent T. Covello, Ph. D. Center for Risk Communication


Vaccine Risk Communication: General Suggestions • • Clarify the issue, source, science Focus on the message rather than the messenger Acknowledge uncertainty Put benefits and risks into perspective (e. g. , a decision not to vaccinate is not risk-free). • Clearly state the main messages • Use words, examples, and analogies that message receivers understand, and view as relevant, to the issue at hand. • Know and use your resources

NATIONAL VACCINE HOTLINE
http: //www. cdc. gov/nip/vacsafe/concerns/gen/of-interest. htm

http: //www. fda. gov/cber/safety. htm

BROCHURES

QUESTIONS?
- Slides: 52