Asst Prof Dr Abdulkarim Y Karim Biochemistry 2
Asst. Prof. Dr. Abdulkarim Y. Karim Biochemistry 2 nd Stage Biology 1 st Lecture Biochemistry (Biological Chemistry) is the chemistry of life or the study of the chemistry of biomolecules and processes occurring in organisms. Biomolecule is any molecule that is produced by a living organism, including large macromolecules such as carbohydrates, lipids, nucleic acids and proteins.
Carbohydrates are • also called saccharides, which means “sugars. ” • composed of the elements C, H and O (CHO). • Carbohydrates are one of the main types of nutrients • They are the most important source of energy for your body. • They form part of the structures of some cells and tissues
Carbohydrates • Are produced by photosynthesis in plants. • Such as glucose are synthesized in plants from CO 2, H 2 O, and energy from the sun. • Are oxidized in living cells to produce CO 2, H 2 O, and energy.
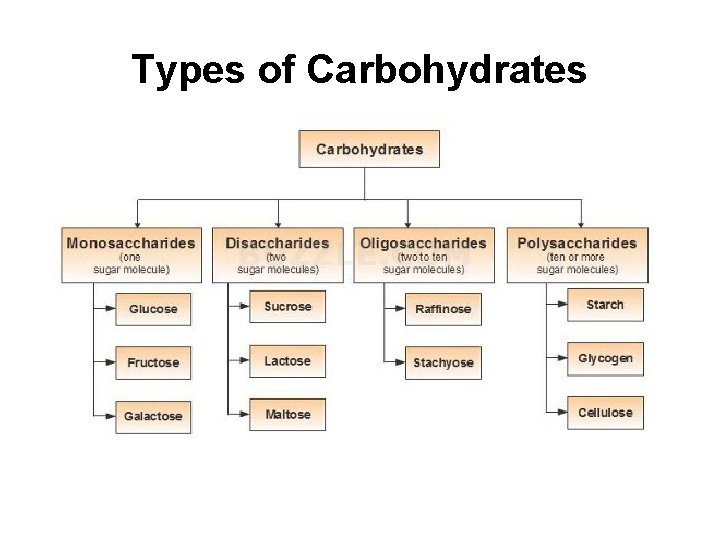
Types of Carbohydrates

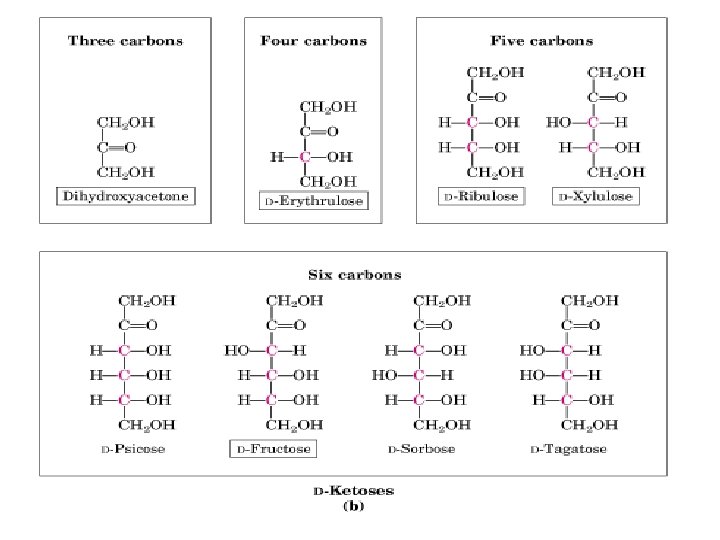
Monosaccharides consist of • 3 -6 carbon atoms typically. • a carbonyl group (aldehyde or ketone). • several hydroxyl groups. • 2 types of monosaccharide structures: Aldoses and ketoses
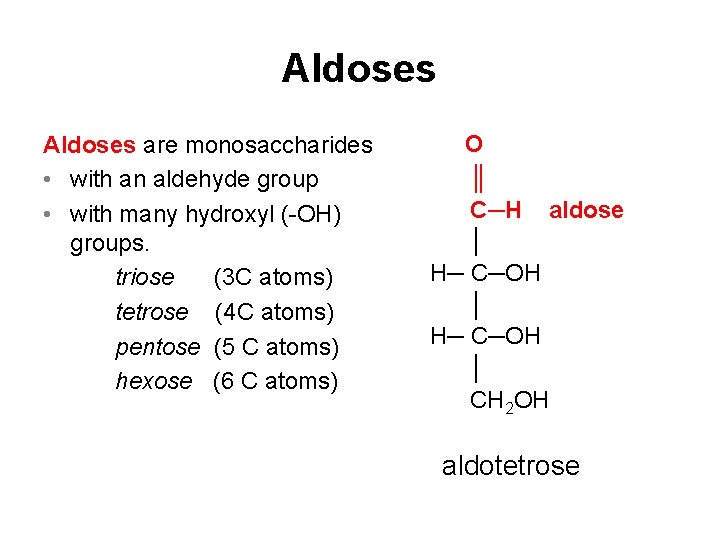
Aldoses are monosaccharides • with an aldehyde group • with many hydroxyl (-OH) groups. triose (3 C atoms) tetrose (4 C atoms) pentose (5 C atoms) hexose (6 C atoms) O ║ C─H aldose │ H─ C─OH │ CH 2 OH aldotetrose
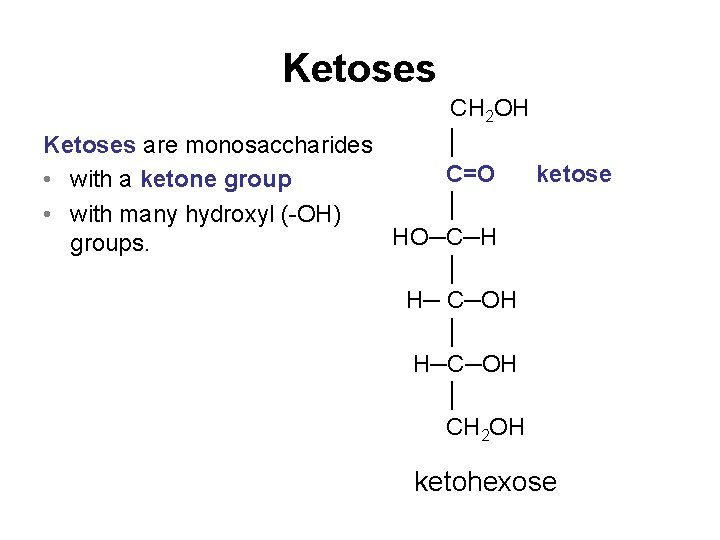
Ketoses CH 2 OH │ Ketoses are monosaccharides C=O ketose • with a ketone group │ • with many hydroxyl (-OH) HO─C─H groups. │ H─ C─OH │ H─C─OH │ CH 2 OH ketohexose

Structures of Monosaccharides
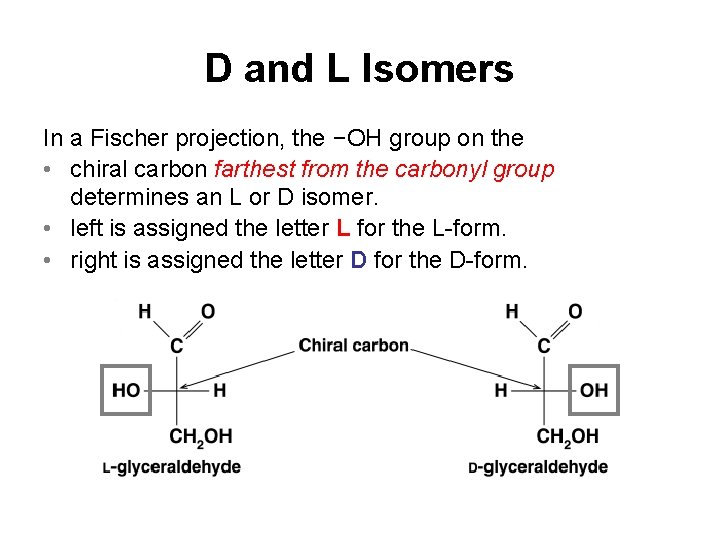
D and L Isomers In a Fischer projection, the −OH group on the • chiral carbon farthest from the carbonyl group determines an L or D isomer. • left is assigned the letter L for the L-form. • right is assigned the letter D for the D-form.
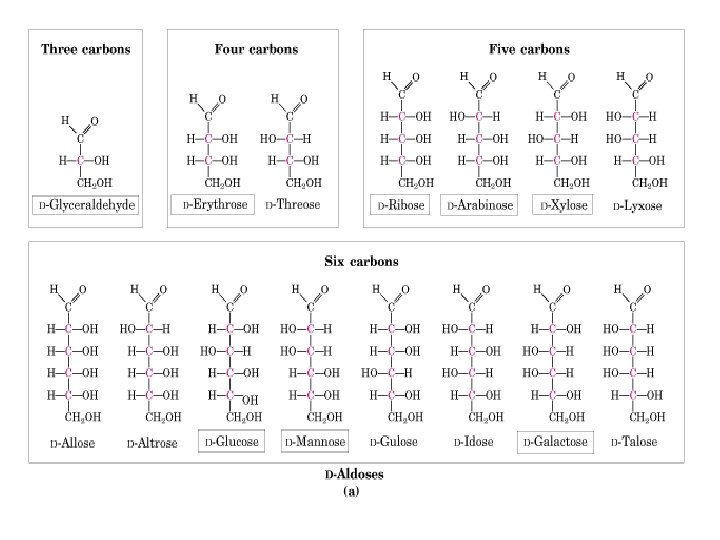
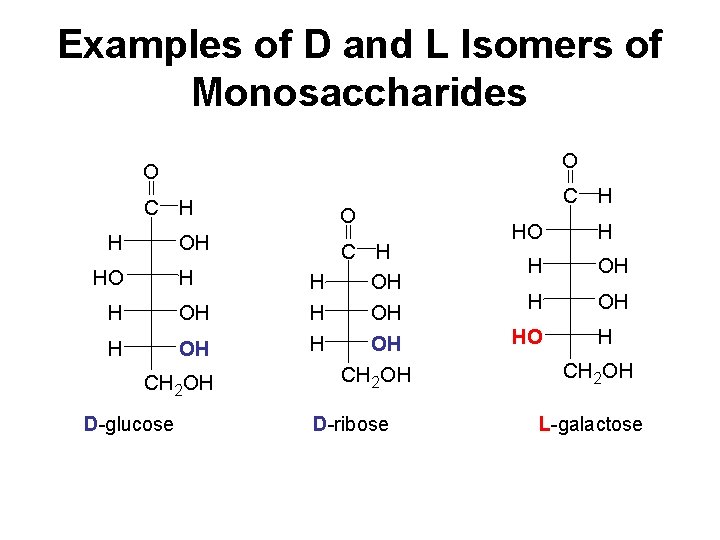
Examples of D and L Isomers of Monosaccharides O O C H H OH HO H H OH CH 2 OH D-glucose C H O C H H OH OH CH 2 OH D-ribose HO H H OH HO H CH 2 OH L-galactose
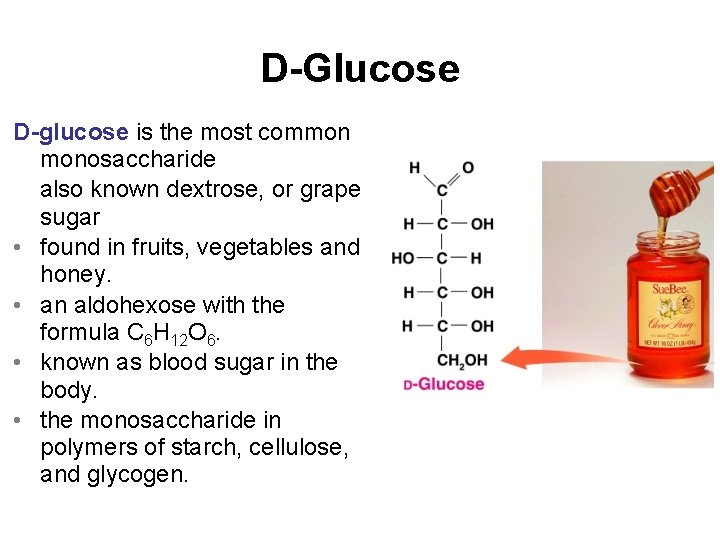
D-Glucose D-glucose is the most common monosaccharide also known dextrose, or grape sugar • found in fruits, vegetables and honey. • an aldohexose with the formula C 6 H 12 O 6. • known as blood sugar in the body. • the monosaccharide in polymers of starch, cellulose, and glycogen.
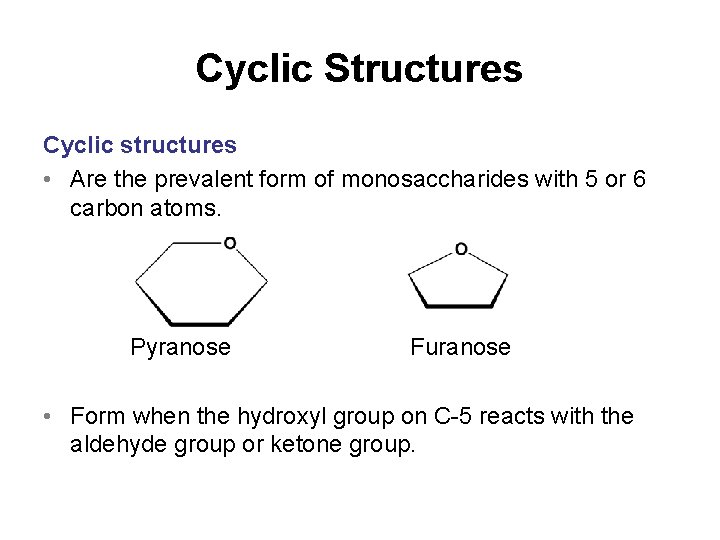
Cyclic Structures Cyclic structures • Are the prevalent form of monosaccharides with 5 or 6 carbon atoms. Pyranose Furanose • Form when the hydroxyl group on C-5 reacts with the aldehyde group or ketone group.
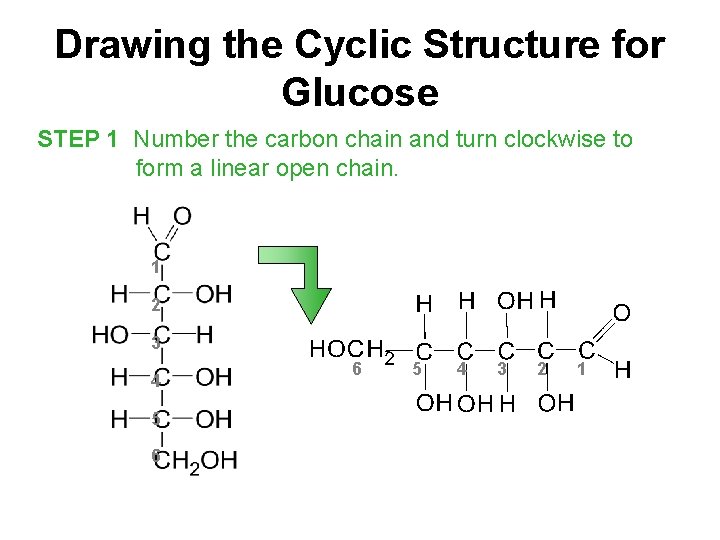
Drawing the Cyclic Structure for Glucose STEP 1 Number the carbon chain and turn clockwise to form a linear open chain. 1 2 3 4 5 6 6 5 4 3 2 1
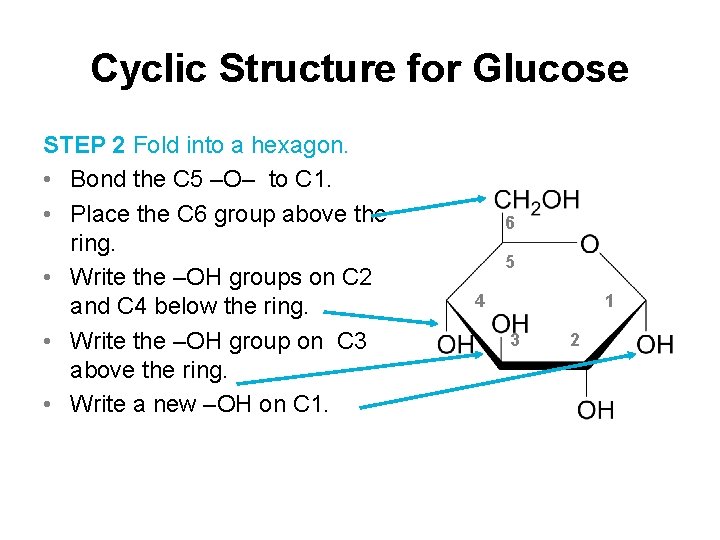
Cyclic Structure for Glucose STEP 2 Fold into a hexagon. • Bond the C 5 –O– to C 1. • Place the C 6 group above the ring. • Write the –OH groups on C 2 and C 4 below the ring. • Write the –OH group on C 3 above the ring. • Write a new –OH on C 1. 6 5 4 1 3 2
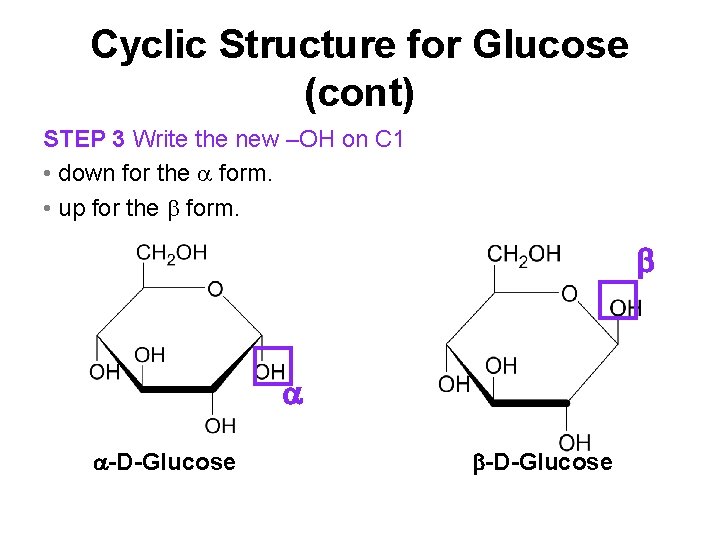
Cyclic Structure for Glucose (cont) STEP 3 Write the new –OH on C 1 • down for the form. • up for the form. -D-Glucose
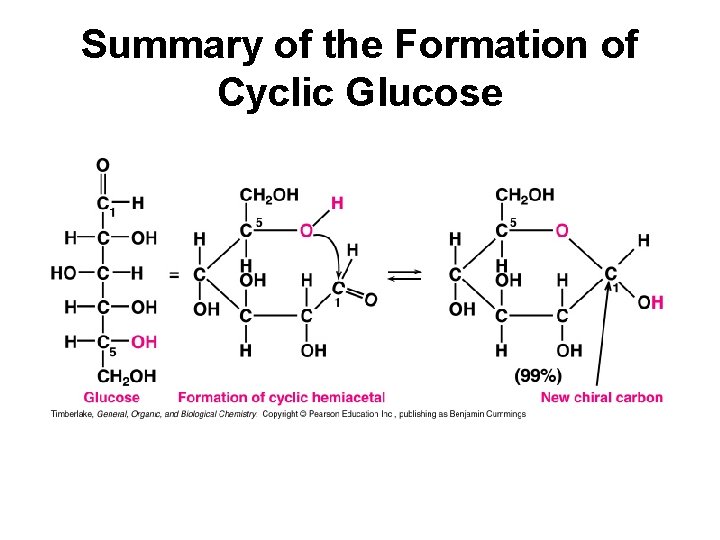
Summary of the Formation of Cyclic Glucose
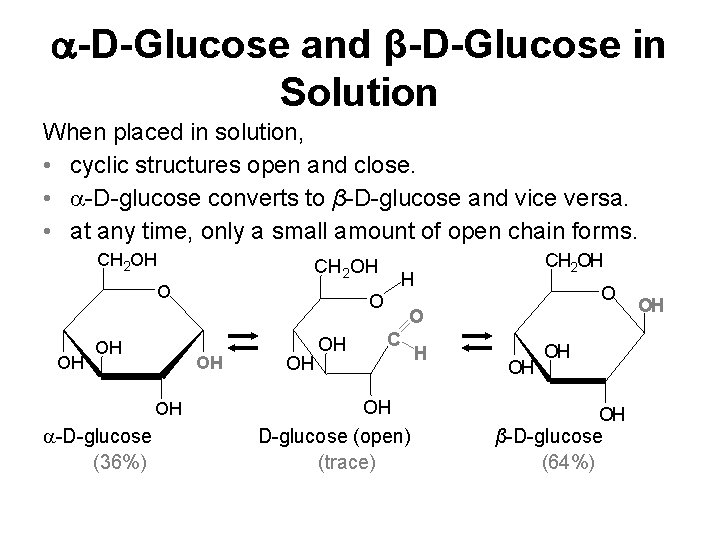
-D-Glucose and β-D-Glucose in Solution When placed in solution, • cyclic structures open and close. • -D-glucose converts to β-D-glucose and vice versa. • at any time, only a small amount of open chain forms. CH 2 OH OH -D-glucose (36%) H O OH OH CH 2 OH O O C OH D-glucose (open) (trace) H OH OH OH β-D-glucose (64%) OH
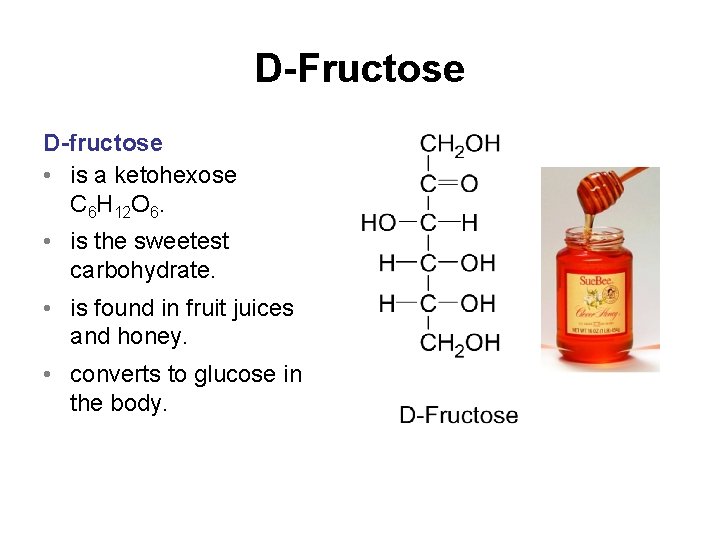
D-Fructose D-fructose • is a ketohexose C 6 H 12 O 6. • is the sweetest carbohydrate. • is found in fruit juices and honey. • converts to glucose in the body.
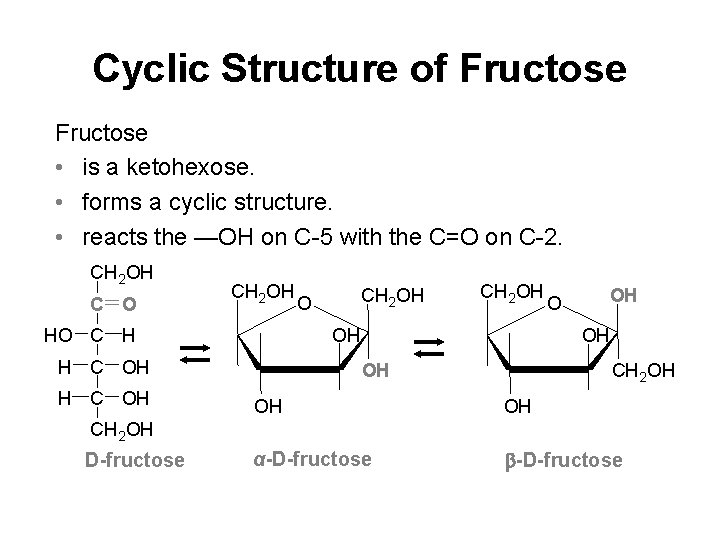
Cyclic Structure of Fructose • is a ketohexose. • forms a cyclic structure. • reacts the —OH on C-5 with the C=O on C-2. CH 2 OH C O CH 2 OH HO C H CH 2 OH OH H C OH CH 2 OH O O OH OH OH CH 2 OH OH OH α-D-fructose CH 2 OH D-fructose
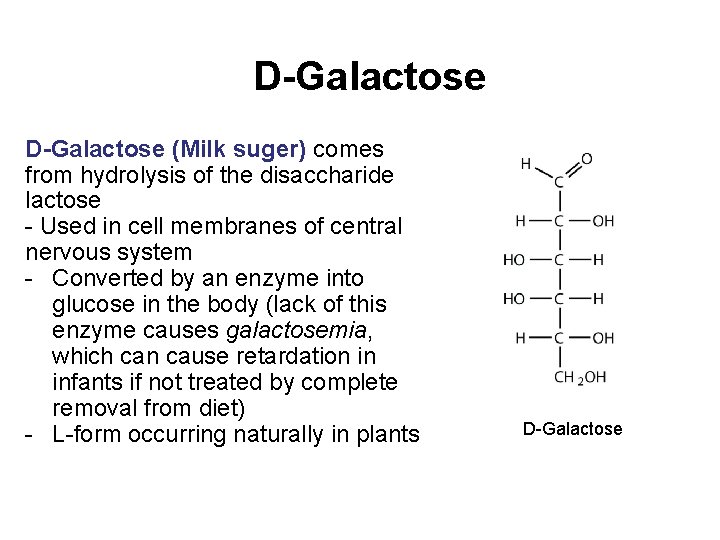
D-Galactose (Milk suger) comes from hydrolysis of the disaccharide lactose - Used in cell membranes of central nervous system - Converted by an enzyme into glucose in the body (lack of this enzyme causes galactosemia, which can cause retardation in infants if not treated by complete removal from diet) - L-form occurring naturally in plants D-Galactose

Physical Properties of Monosaccharides • Most monosaccharides have a sweet taste. • They are solids at room temperature. • They are extremely soluble in water: – Despite their high molecular weights, the presence of large numbers of OH groups make the monosaccharides much more water soluble than most molecules of similar MW.
- Slides: 23