Assignment Read 5 6 up to sample 238


- Slides: 14


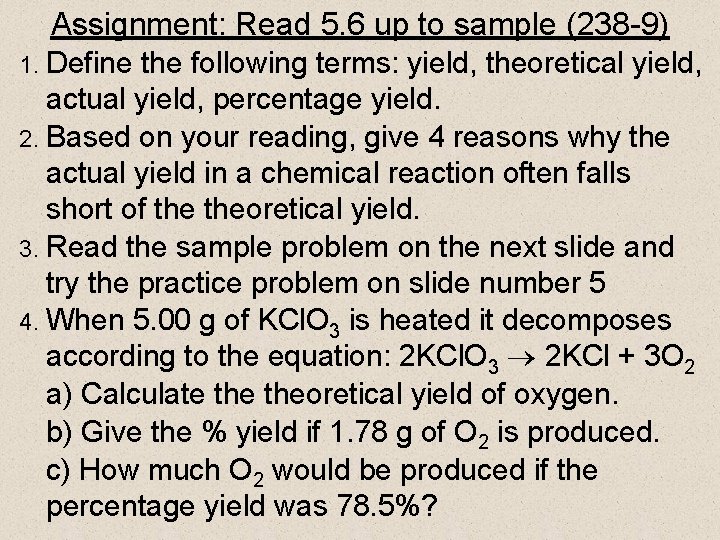
Assignment: Read 5. 6 up to sample (238 -9) 1. Define the following terms: yield, theoretical yield, actual yield, percentage yield. 2. Based on your reading, give 4 reasons why the actual yield in a chemical reaction often falls short of theoretical yield. 3. Read the sample problem on the next slide and try the practice problem on slide number 5 4. When 5. 00 g of KCl. O 3 is heated it decomposes according to the equation: 2 KCl. O 3 2 KCl + 3 O 2 a) Calculate theoretical yield of oxygen. b) Give the % yield if 1. 78 g of O 2 is produced. c) How much O 2 would be produced if the percentage yield was 78. 5%?

Answers 1) Yield: the amount of product Theoretical yield: the amount of product we expect, based on stoichiometric calculations Actual yield: amount of product from a procedure or experiment (this is given in the question) actual yield Percent yield: x 100% theoretical yield 2) • Not all product is recovered (e. g. spattering) • Reactant impurities (e. g. weigh out 100 g of chemical which has 20 g of junk) • A side reaction occurs (e. g. Mg. O vs. Mg 3 N 2) • The reaction does not go to completion

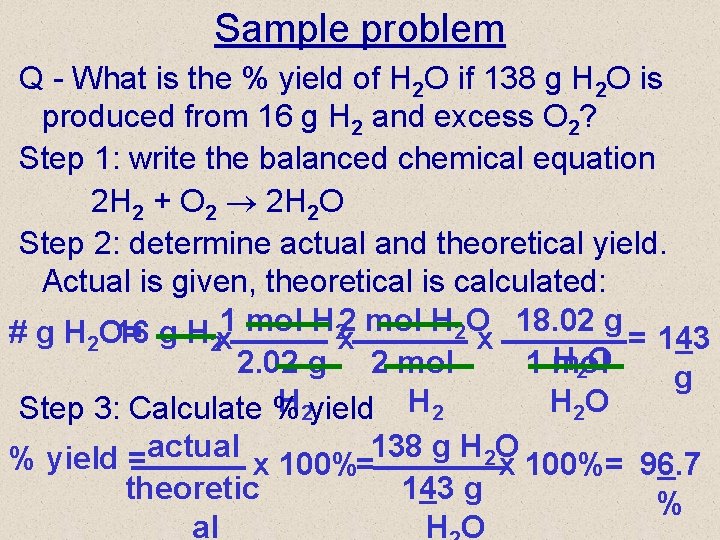
Sample problem Q - What is the % yield of H 2 O if 138 g H 2 O is produced from 16 g H 2 and excess O 2? Step 1: write the balanced chemical equation 2 H 2 + O 2 2 H 2 O Step 2: determine actual and theoretical yield. Actual is given, theoretical is calculated: 2 mol H 2 O 18. 02 g # g H 2 O= 16 g H 2 x 1 mol H 2 x x = 143 H 2 O 2. 02 g 2 mol 1 mol g H 2 yield H 2 O Step 3: Calculate % % yield =actual x 100%=138 g H 2 O x 100%= 96. 7 theoretic 143 g % al HO

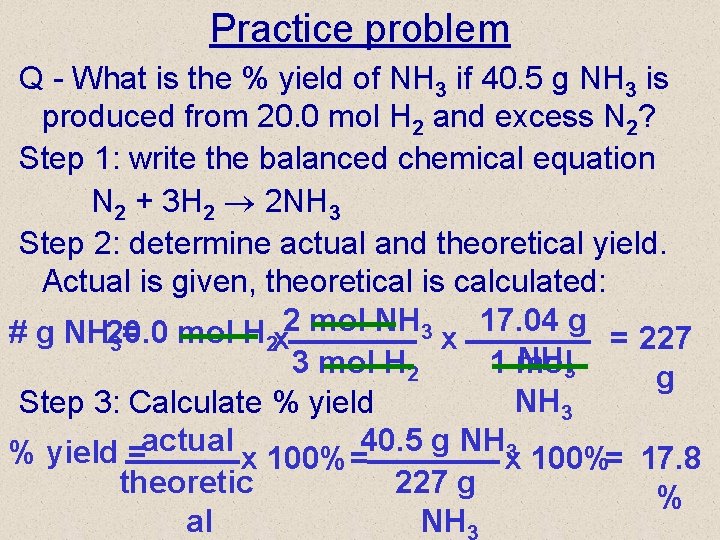
Practice problem Q - What is the % yield of NH 3 if 40. 5 g NH 3 is produced from 20. 0 mol H 2 and excess N 2? Step 1: write the balanced chemical equation N 2 + 3 H 2 2 NH 3 Step 2: determine actual and theoretical yield. Actual is given, theoretical is calculated: 2 mol NH 3 17. 04 g # g NH 20. 0 = mol H x = 227 3 2 x NH 3 3 mol H 2 1 mol g NH 3 Step 3: Calculate % yield =actual x 100% =40. 5 g NHx 3 100%= 17. 8 theoretic 227 g % al NH 3

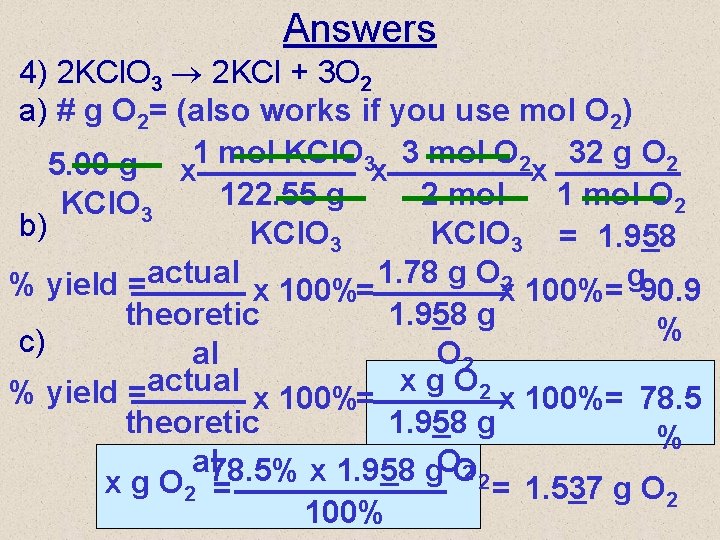
Answers 4) 2 KCl. O 3 2 KCl + 3 O 2 a) # g O 2= (also works if you use mol O 2) 5. 00 g x 1 mol KCl. O 3 x 3 mol O 2 x 32 g O 2 1 mol O 2 122. 55 g 2 mol KCl. O 3 b) KCl. O 3 = 1. 958 % yield =actual x 100%= 1. 78 g Ox 2 100%= g 90. 9 theoretic 1. 958 g % c) al O 2 % yield =actual x 100%= x g O 2 x 100%= 78. 5 theoretic 1. 958 g % al 78. 5% x 1. 958 g. OO 2 2 = 1. 537 g O x g O 2 = 2 100%

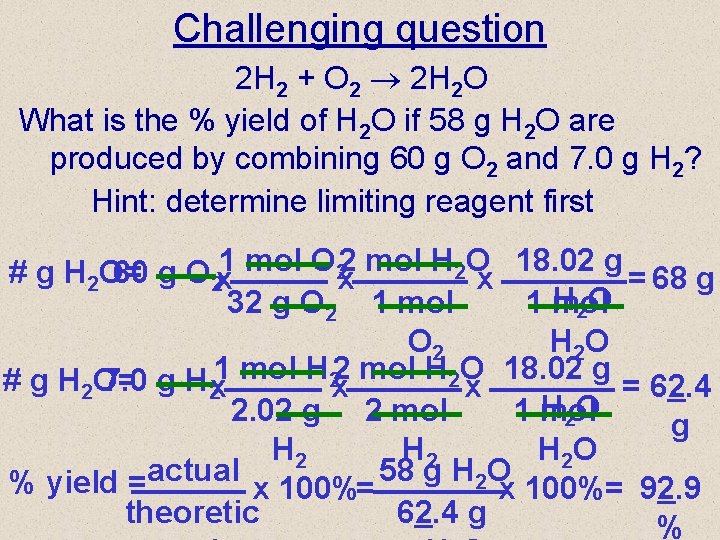
Challenging question 2 H 2 + O 2 2 H 2 O What is the % yield of H 2 O if 58 g H 2 O are produced by combining 60 g O 2 and 7. 0 g H 2? Hint: determine limiting reagent first 2 mol H 2 O 18. 02 g # g H 2 O= 60 g O 2 x 1 mol O 2 x x = 68 g H 2 O 32 g O 2 1 mol O 2 H 2 O 2 mol H 2 O 18. 02 g # g H 2 O= 7. 0 g H 2 x 1 mol H 2 x x = 62. 4 H 2 O 2. 02 g 2 mol 1 mol g H 2 H 2 O % yield =actual x 100%= 58 g H 2 Ox 100%= 92. 9 theoretic 62. 4 g %

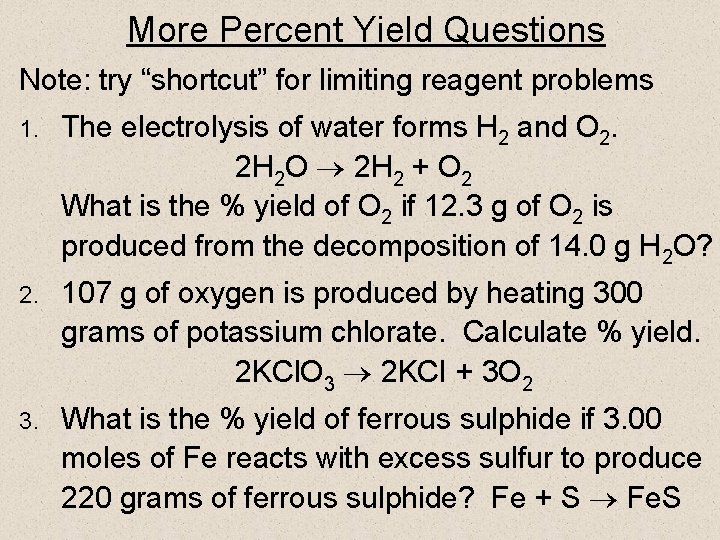
More Percent Yield Questions Note: try “shortcut” for limiting reagent problems 1. The electrolysis of water forms H 2 and O 2. 2 H 2 O 2 H 2 + O 2 What is the % yield of O 2 if 12. 3 g of O 2 is produced from the decomposition of 14. 0 g H 2 O? 2. 107 g of oxygen is produced by heating 300 grams of potassium chlorate. Calculate % yield. 2 KCl. O 3 2 KCI + 3 O 2 3. What is the % yield of ferrous sulphide if 3. 00 moles of Fe reacts with excess sulfur to produce 220 grams of ferrous sulphide? Fe + S Fe. S

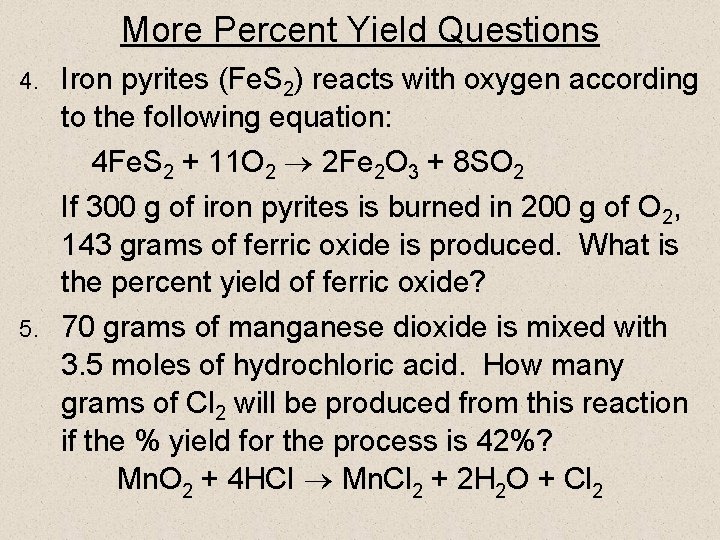
More Percent Yield Questions Iron pyrites (Fe. S 2) reacts with oxygen according to the following equation: 4 Fe. S 2 + 11 O 2 2 Fe 2 O 3 + 8 SO 2 If 300 g of iron pyrites is burned in 200 g of O 2, 143 grams of ferric oxide is produced. What is the percent yield of ferric oxide? 5. 70 grams of manganese dioxide is mixed with 3. 5 moles of hydrochloric acid. How many grams of Cl 2 will be produced from this reaction if the % yield for the process is 42%? Mn. O 2 + 4 HCI Mn. Cl 2 + 2 H 2 O + Cl 2 4.

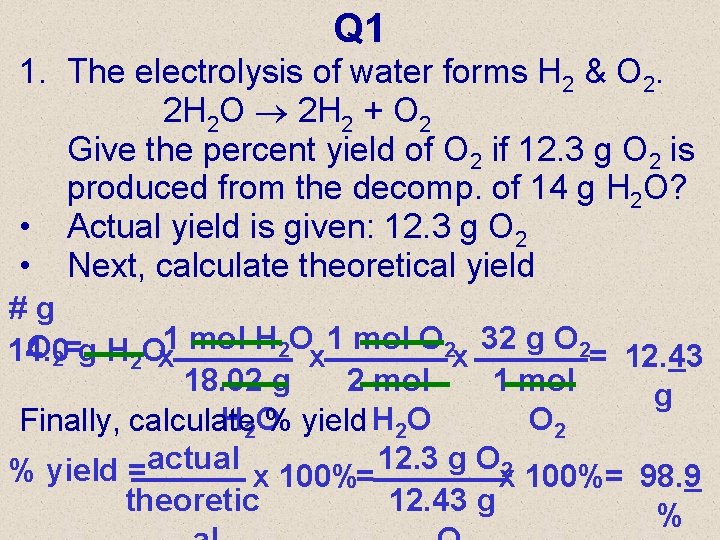
Q 1 1. The electrolysis of water forms H 2 & O 2. 2 H 2 O 2 H 2 + O 2 Give the percent yield of O 2 if 12. 3 g O 2 is produced from the decomp. of 14 g H 2 O? • Actual yield is given: 12. 3 g O 2 • Next, calculate theoretical yield #g O 2=g H 2 Ox 1 mol O 2 x 32 g O 2= 12. 43 14. 0 18. 02 g 2 mol 1 mol g H 2 O O 2 Finally, calculate % yield H 2 O % yield =actual x 100%= 12. 3 g Ox 2 100%= 98. 9 theoretic 12. 43 g %

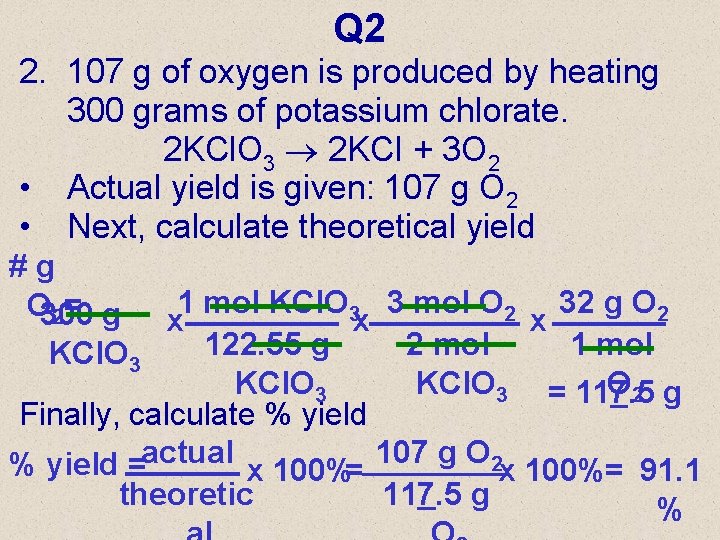
Q 2 2. 107 g of oxygen is produced by heating 300 grams of potassium chlorate. 2 KCl. O 3 2 KCI + 3 O 2 • Actual yield is given: 107 g O 2 • Next, calculate theoretical yield #g 1 mol KCl. O 3 3 mol O 2 32 g O 2 O 300 2= g x x x 122. 55 g 2 mol 1 mol KCl. O 3 = 117. 5 O 2 g Finally, calculate % yield =actual x 100%= 107 g O 2 x 100%= 91. 1 theoretic 117. 5 g %

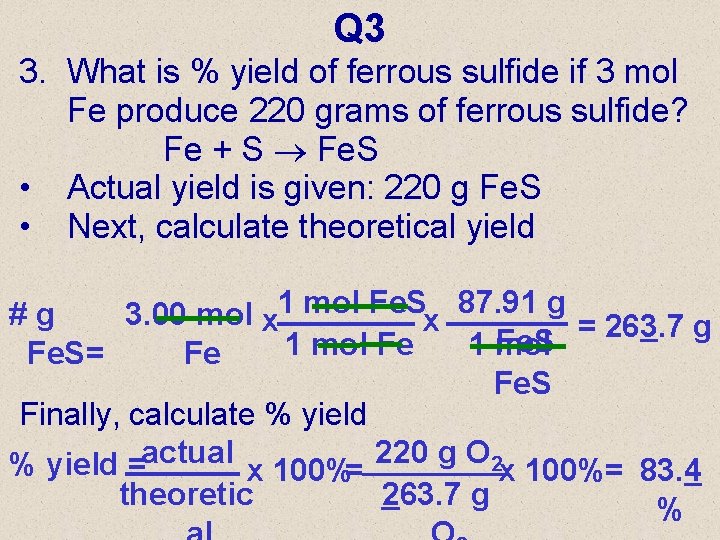
Q 3 3. What is % yield of ferrous sulfide if 3 mol Fe produce 220 grams of ferrous sulfide? Fe + S Fe. S • Actual yield is given: 220 g Fe. S • Next, calculate theoretical yield #g 3. 00 mol x 1 mol Fe. Sx 87. 91 g = 263. 7 g Fe. S 1 mol Fe. S= Fe Fe. S Finally, calculate % yield =actual x 100%= 220 g O 2 x 100%= 83. 4 theoretic 263. 7 g %

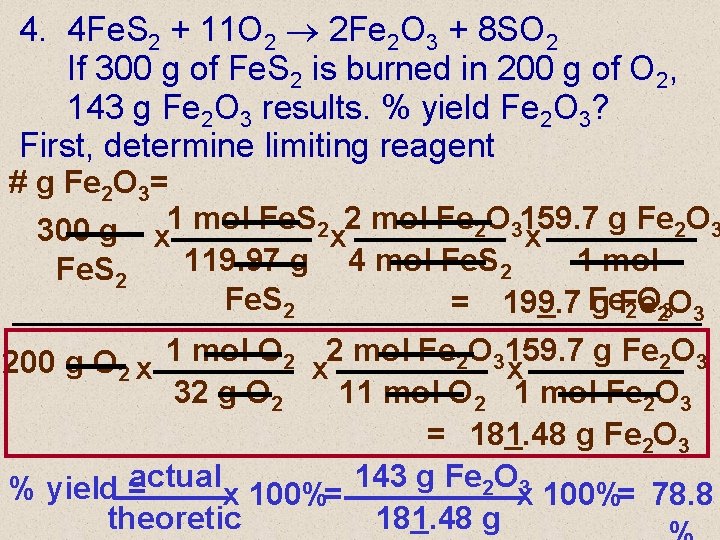
4. 4 Fe. S 2 + 11 O 2 2 Fe 2 O 3 + 8 SO 2 If 300 g of Fe. S 2 is burned in 200 g of O 2, 143 g Fe 2 O 3 results. % yield Fe 2 O 3? First, determine limiting reagent # g Fe 2 O 3= g Fe 2 O 3 300 g x 1 mol Fe. S 2 x 2 mol Fe 2 O 3159. 7 x 119. 97 g 4 mol Fe. S 2 1 mol Fe. S 2 = 199. 7 Fe g Fe 2 O 23 O 3 g Fe 2 O 3 200 g O 2 x 1 mol O 2 x 2 mol Fe 2 O 3159. 7 x 32 g O 2 11 mol O 2 1 mol Fe 2 O 3 = 181. 48 g Fe 2 O 3 actual 143 g Fe 2 O 3 % yield = x 100%= 78. 8 theoretic 181. 48 g

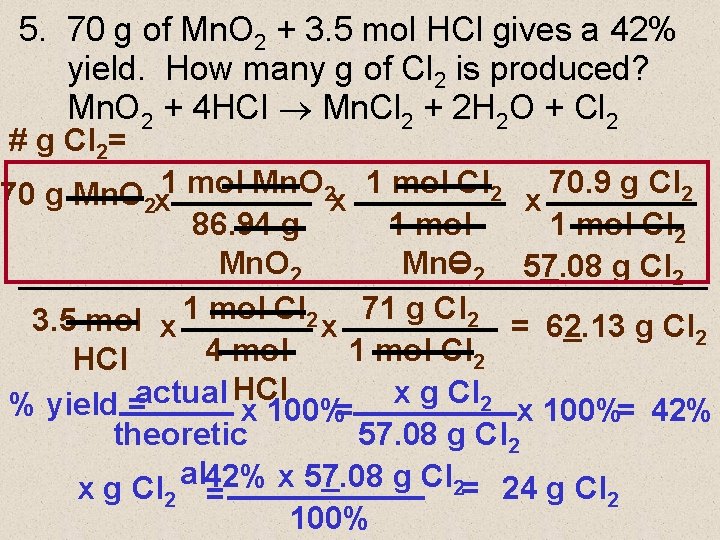
5. 70 g of Mn. O 2 + 3. 5 mol HCl gives a 42% yield. How many g of Cl 2 is produced? Mn. O 2 + 4 HCI Mn. Cl 2 + 2 H 2 O + Cl 2 # g Cl 2= 70 g Mn. O 2 x 1 mol Cl 2 x 70. 9 g Cl 2 86. 94 g 1 mol Cl 2 Mn. O = 2 57. 08 g Cl 2 3. 5 mol x 1 mol Cl 2 x 71 g Cl 2 = 62. 13 g Cl 2 4 mol 1 mol Cl 2 HCl actual x g Cl 2 % yield = x 100%= 42% theoretic 57. 08 g Cl 2 al x 57. 08 g Cl 2= 24 g Cl x g Cl 2 42% = 2 100%