Assignment n Read page 158 165 on simple














- Slides: 16

Assignment: n Read page 158 -165 on simple ions!!!

And now it’s time for…. . stupid halloween riddles!! n n Guess the riddle—win a candy!! They come fast and furious!!

The Magic of Chemistry--Bonding n n n There about 100 elements, but there are millions of different compounds. How is this possible? Through the “miracle” of chemical bonding! Bonding is the process where atoms or molecules combine or bond together to form new substances. n How can you possibly keep all of these chemicals straight? You must have a naming system!!!

The two prior statements put together mean… 1. You must learn how to name compounds. 2. You must learn how bonding works. 3. You must learn them together!! 4. First, let’s start with some basics.

The first rule about bonding is to learn that some atoms don’t bond!! Can you guess which ones they are? That’s right—it’s the noble gases--Helium, Neon, Argon, Krypton, Xenon, and Radon They exist by themselves and so they are “monatomic”, meaning “one atom”

Why do they exist by themselves? Remember the octet rule? Atoms are stable and therefore “happy” if they have 8 electrons in their outermost energy level. The noble gases are already stable. ***This is called the “noble gas configuration” Exception!! The small elements are stable with only two in their outer level because that is all they can fit! Which are they?

Most Atoms do Bond!!! Here we see the German “blimp” the Hindenberg, literally blowing up. Its’ hydrogen atoms were combining with oxygen atoms. When they do, they are held together by a force it is called “bonding”. (think of glue)

Bonds form during chemical reactions!! Here’s a cool one-Pharoahs Snake. (Mercury thiocyanate—”burning”)

The squealing jelly baby

The screaming jelly baby explained

There are two main types of bonds that form when atoms join. They are: 1. molecular—also called covalent. These are where electrons are shared. 2. Ionic—called ionic bonds—These are where electrons are actually transferred. But the goal is the same for each atom—to have a stable arrangement of electrons in its outer ring!! When molecular bonds are formed the substance is called a molecule. When ionic bonds are formed, the substance is not called a molecule—it is called a formula unit.

Ion formation—a brief review of some previous facts you’ve learned n n Positive ions are formed because an atom or molecule has lost an electron (more plusses than minuses) Positive ions are called cations (cat ions) Negative ions are formed because an atom or molecule has gained an electron (more minus than plus) Negative ions are called anions (an ions)

The octet magic n Two ways: gain one, two, or three for a full octet: or lose one, two, or three and “leave” a full octet. Look for this……. Add one and you have eight! Seven electrons in the outer ring Or you could have: Lose one and have eight “left” One electron in the outer ring Or you could have: Add two and have eight!! Six electrons in the outer ring Lose two and have eight “left” Two electrons in the outer ring Add three and have eight Five electrons in the outer ring Lose three and have eight “left” Three electrons in the outer ring

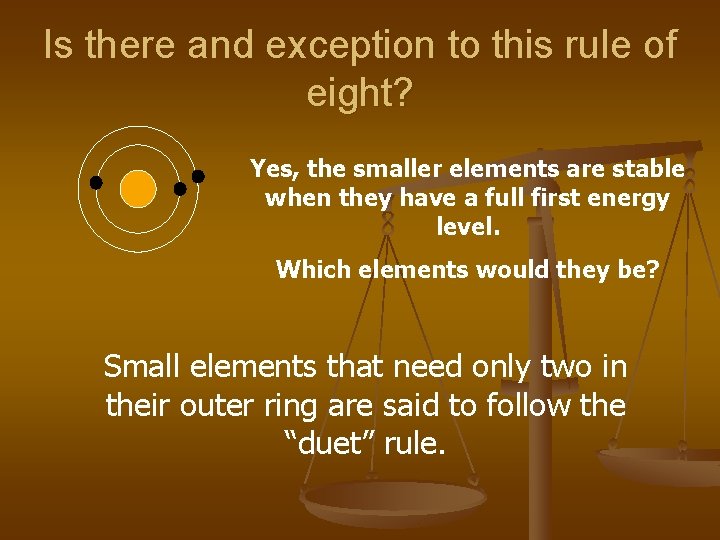
Is there and exception to this rule of eight? Yes, the smaller elements are stable when they have a full first energy level. Which elements would they be? Small elements that need only two in their outer ring are said to follow the “duet” rule.

What exactly happens when an ion forms? The chemical reaction of sodium and chlorine How ions form in a chemical reaction between sodium and chlorine-part deux What does this reaction actually look like?

One last thing on ions: Depending on the atom, 1, 2 , 3 or more electrons can be gained or lost. So, when an atom loses one we say it has a plus one charge—which is written with the symbol and a little number in the upper right indicating whether it is a plus charge (lost electrons) or a minus charge (gained electrons) Examples: Na +1 Ca +2 Fe +2 Al +3 Cl -1 S -2 P -3