Assigning Oxidation Numbers Rules HalfRxn 8 Rules for

Assigning Oxidation Numbers Rules & Half-Rxn

8 Rules for Oxidation Numbers 1. of a free, uncombined element = 0. Na He O 2 N 2 S 8 Cl 2 2. of a monatomic ion = charge on ion. +2 -1 +3 P Ca = +2. Cl = -1. Al = +3. Remember: Ions occur in ionic compounds: Ca. Cl 2, Al(NO 3)3, etc.

8 Rules for Oxidation Numbers 3. Fluorine is always -1. CF 4 4. Hydrogen is nearly always +1, except when it’s bonded to a metal. Then it’s -1. H 2 O, HNO 3, H 2 SO 4 Li. H Ca. H 2 Na. H

8 Rules for Oxidation Numbers 5. Oxygen is nearly always -2 except when it’s… -Bonded to fluorine, where O is +2 OF 2 -In the peroxide ion, where O is -1. eg. H 2 O 2 and Na 2 O 2 O 22 -

8 Rules for Oxidation Numbers 6. The sum of oxidation numbers in a neutral compound is 0. H 2 O CO 2 NO SO 3 7. The sum of oxidation numbers in a polyatomic ion = charge of the ion. Sum in SO 42 - = -2. Sum in NO 3 - = -1.

8 Rules for Oxidation Numbers 8. In covalent compounds, the oxidation number of the more electronegative atom is the negative charge it would have if it was an ion. *NH 3: N = -3, H = +1. Si. Cl 4: Si = +4, Cl = -1.

+4 +3 +2 +1 0 -1 -2 -3 -4 2) And if you’re lucky you strike oil & it shoots up 1) You dig down with an oil rig
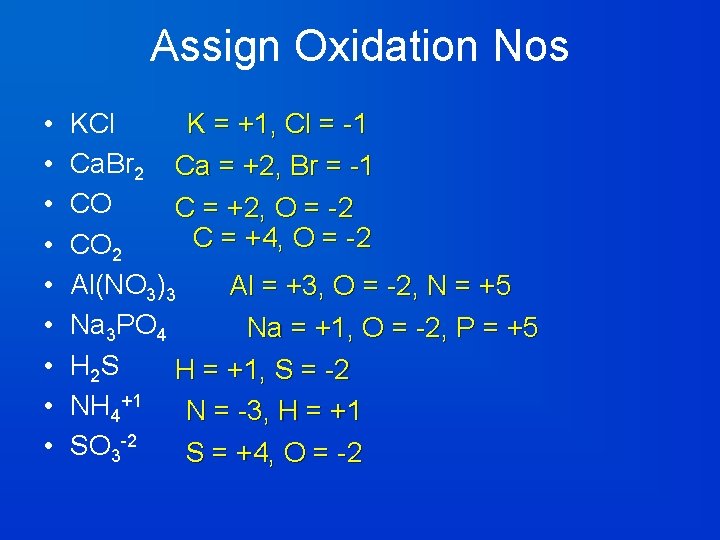
Assign Oxidation Nos • • • K = +1, Cl = -1 KCl Ca. Br 2 Ca = +2, Br = -1 CO C = +2, O = -2 C = +4, O = -2 CO 2 Al(NO 3)3 Al = +3, O = -2, N = +5 Na 3 PO 4 Na = +1, O = -2, P = +5 H 2 S H = +1, S = -2 NH 4+1 N = -3, H = +1 SO 3 -2 S = +4, O = -2

Electrons are Negative! • Why do we use the word “reduced” when electrons are gained? Look at how the oxidation number changes. For example, if Cl gains an electron it becomes Cl-1. The oxidation number decreased from 0 to -1. The oxidation number was reduced.
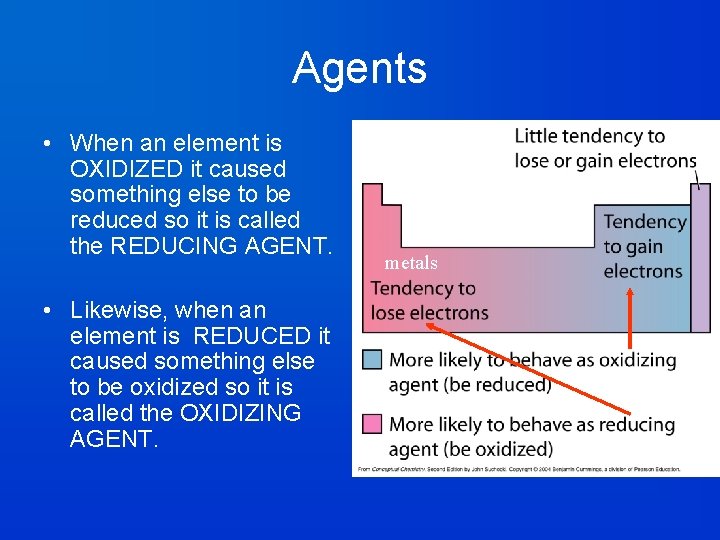
Agents • When an element is OXIDIZED it caused something else to be reduced so it is called the REDUCING AGENT. • Likewise, when an element is REDUCED it caused something else to be oxidized so it is called the OXIDIZING AGENT. Non-metals

Vocabulary Interlude • Oxidizing Agent: Is itself reduced. Accepts electrons from something else – aids oxidation for another species. • Reducing Agent: Is itself oxidized. • Loses electrons to something else – aids reduction for another species.
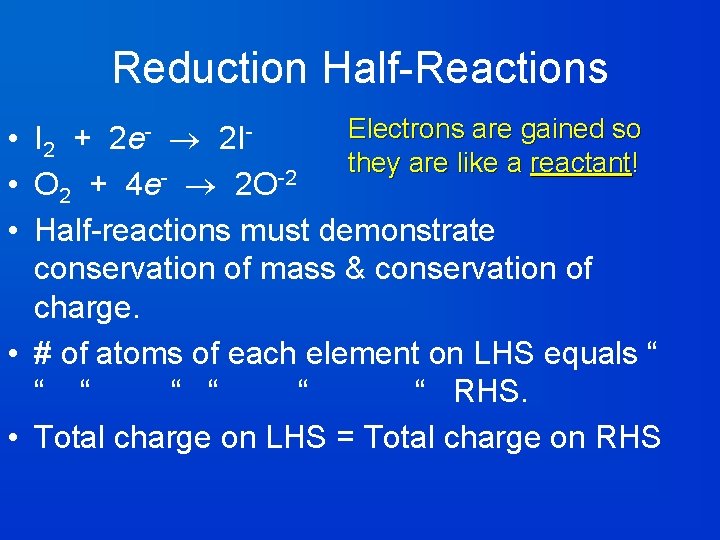
Reduction Half-Reactions Electrons are gained so • I 2 + 2 e- 2 Ithey are like a reactant ! • O 2 + 4 e- 2 O-2 • Half-reactions must demonstrate conservation of mass & conservation of charge. • # of atoms of each element on LHS equals “ “ “ “ RHS. • Total charge on LHS = Total charge on RHS
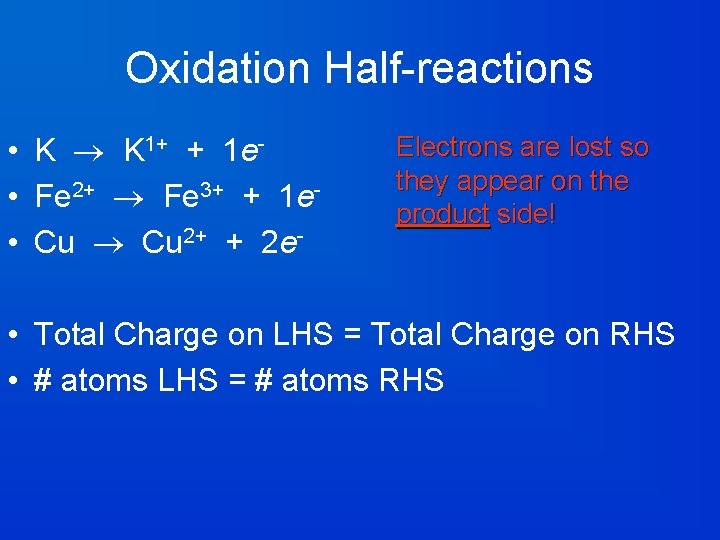
Oxidation Half-reactions • K K 1+ + 1 e • Fe 2+ Fe 3+ + 1 e • Cu 2+ + 2 e- Electrons are lost so they appear on the product side! • Total Charge on LHS = Total Charge on RHS • # atoms LHS = # atoms RHS
- Slides: 13