ASSESSMENT OF MAJOR FACTORS AFFECTING TRIPLOID INDUCTION USING




- Slides: 6

ASSESSMENT OF MAJOR FACTORS AFFECTING TRIPLOID INDUCTION USING HYDROSTATIC PRESSURE IN THE EASTERN OYSTER (Crassostrea virginica) Name: Ian Sewell (MSc candidate) Supervisor: Dr. Sarah Stewart - Clark Date: January 25, 2018 1

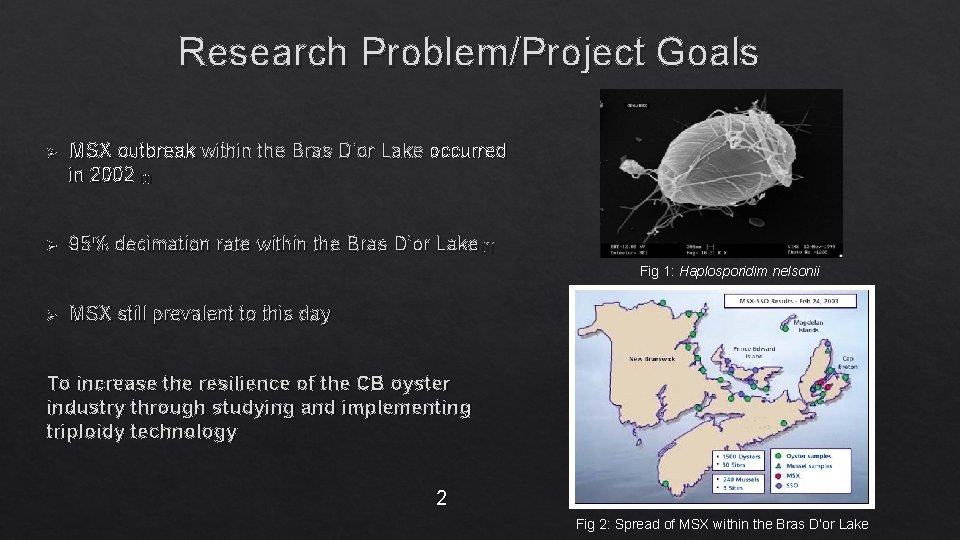
Research Problem/Project Goals Ø MSX outbreak within the Bras D’or Lake occurred in 2002 [1] Ø 95% decimation rate within the Bras D’or Lake [1] Fig 1: Haplosporidim nelsonii Ø MSX still prevalent to this day To increase the resilience of the CB oyster industry through studying and implementing triploidy technology 2 Fig 2: Spread of MSX within the Bras D’or Lake

Potential Solution Ø MSX initial outbreak in the US dated back to the 1950 s [2] Ø The effects of MSX completely eradicated through polyploidy and breeding [2] Ø Transfer of resistant/triploid/tetraploid oysters– Not a possibility Ø Oysters in Canada are uniquely adapted to Canadian environmental and disease pressures Ø Research Approach: Learn from them and implement these practises 3

Method & Results 4 Triploidy experiment Deliverables 3 x 3 Multifactorial approach - 9 treatment Ø Triploid induction success in all treatments Ø Up to 44% triploid induction rate Ø No significant differences among treatments (P=0. 229) Ø Unintentional tetraploid success through late PB timing (70% 1 st Polar body) Ø Successful triploid groups deployed in the Bras D’or Lake for 2 N/ 3 N growth analysis combinations To determine the effect of: 1. Pressure intensity (3 levels): 6000, 7500 PSI 2. Pressure duration (3 levels): 3, 5, 10 mins 3. FIXED treatment initiation stage: 60% 1 st Polar body [3] Larval ploidy level checked using flow cytometry (ABC hatcheries, VIMS) [4]

Acknowledgements & References I’d like to thank the AANS, DAL Aquaculture staff , VIMS – ABC Hatchery staff, my supervisor – Dr. Sarah Stewart-Clark, Scott Jeffrey, my academic committee, and all the involved farms for their assistance and participation throughout the project. References [1] Ford, S. E. and Haskin, H. H. 1982. History and Epizootiology of Haplosporidium nelsoni (MSX), an oyster pathogen in Delaware Bay, 1957 – 1980. J. Invertebr. Pathol. 40: 118 – 141 [2] Aquaculture Genetics and Breeding Technology Center. 2009. Virginia Institute of Marine Science, School of Marine Science, College of William and Mary. pp 28. [3] Allen Jr, S. K. , Downing, S. L. , Chew, K. K. 1989. Hatchery manual for producing triploid oysters. University of Washington Press, Seattle, WA, USA. [4] Allen Jr, S. K. 1983. Flow cytometry: assaying experimental polyploid fish and shellfish. Aquaculture. 33: 317 – 328 5

6 End of Presentation