Assessment and Prognosis of Patients with IPF Educational




- Slides: 33

Assessment and Prognosis of Patients with IPF

Educational Activity Learning Objective • Utilize indicators of disease status to diagnose, assess, and manage patients with IPF

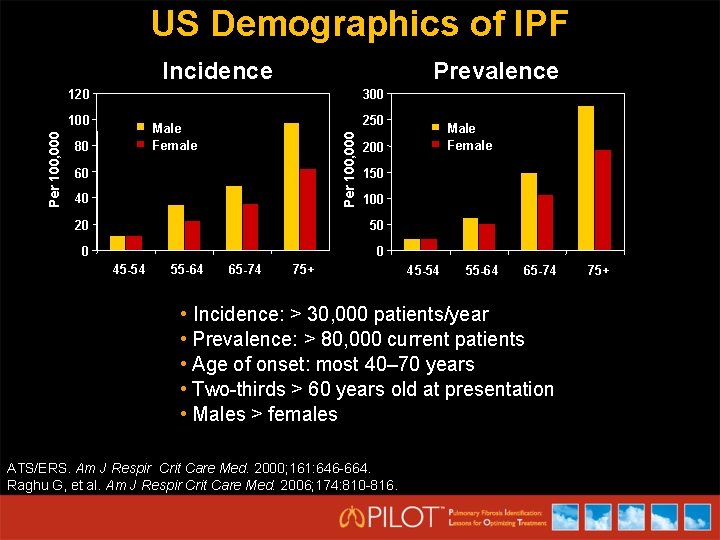
US Demographics of IPF Incidence Prevalence 120 300 250 Male Female 80 Per 100, 000 100 60 40 200 150 100 20 50 0 0 45 -54 55 -64 65 -74 Male Female 75+ 45 -54 55 -64 65 -74 • Incidence: > 30, 000 patients/year • Prevalence: > 80, 000 current patients • Age of onset: most 40– 70 years • Two-thirds > 60 years old at presentation • Males > females ATS/ERS. Am J Respir Crit Care Med. 2000; 161: 646 -664. Raghu G, et al. Am J Respir Crit Care Med. 2006; 174: 810 -816. 75+

Risk Factors for IPF • Risk factors – Familial – Smoking • Possible associated factors – Environment (eg, wood or metal dust) – Gastroesophageal reflux disease (GERD) – Infectious agents ATS/ERS. Am J Respir Crit Care Med. 2000; 161: 646 -664. Raghu G, et al. Am J Respir Crit Care Med. 2006; 174: 810 -816.

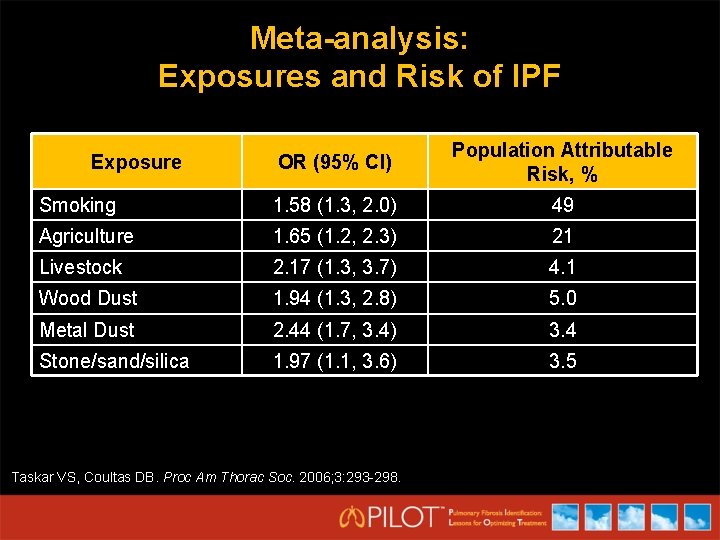
Meta-analysis: Exposures and Risk of IPF OR (95% CI) Population Attributable Risk, % Smoking 1. 58 (1. 3, 2. 0) 49 Agriculture 1. 65 (1. 2, 2. 3) 21 Livestock 2. 17 (1. 3, 3. 7) 4. 1 Wood Dust 1. 94 (1. 3, 2. 8) 5. 0 Metal Dust 2. 44 (1. 7, 3. 4) 3. 4 Stone/sand/silica 1. 97 (1. 1, 3. 6) 3. 5 Exposure Taskar VS, Coultas DB. Proc Am Thorac Soc. 2006; 3: 293 -298.

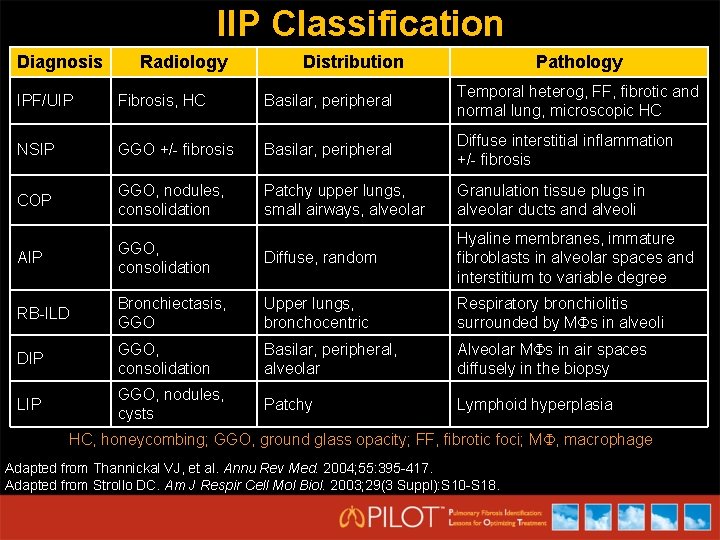
IIP Classification Diagnosis Radiology Distribution Pathology IPF/UIP Fibrosis, HC Basilar, peripheral Temporal heterog, FF, fibrotic and normal lung, microscopic HC NSIP GGO +/- fibrosis Basilar, peripheral Diffuse interstitial inflammation +/- fibrosis COP GGO, nodules, consolidation Patchy upper lungs, small airways, alveolar Granulation tissue plugs in alveolar ducts and alveoli AIP GGO, consolidation Diffuse, random Hyaline membranes, immature fibroblasts in alveolar spaces and interstitium to variable degree RB-ILD Bronchiectasis, GGO Upper lungs, bronchocentric Respiratory bronchiolitis surrounded by M s in alveoli DIP GGO, consolidation Basilar, peripheral, alveolar Alveolar M s in air spaces diffusely in the biopsy LIP GGO, nodules, cysts Patchy Lymphoid hyperplasia HC, honeycombing; GGO, ground glass opacity; FF, fibrotic foci; M , macrophage Adapted from Thannickal VJ, et al. Annu Rev Med. 2004; 55: 395 -417. Adapted from Strollo DC. Am J Respir Cell Mol Biol. 2003; 29(3 Suppl): S 10 -S 18.

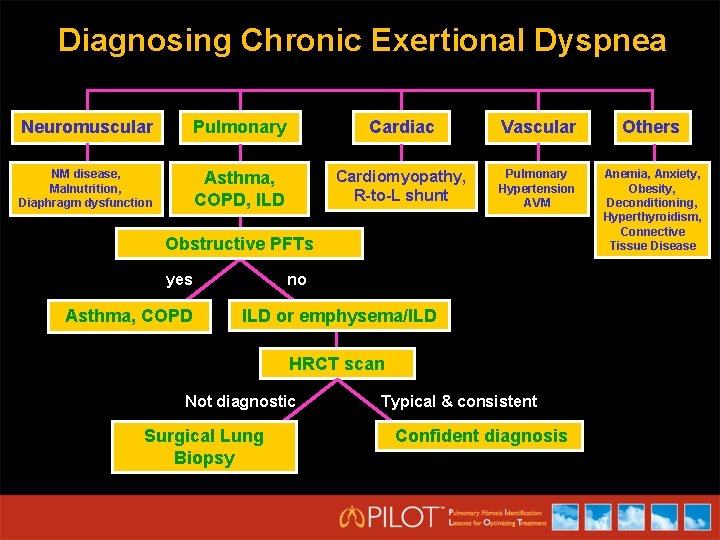
Diagnosing Chronic Exertional Dyspnea Neuromuscular Pulmonary Cardiac Vascular Others NM disease, Malnutrition, Diaphragm dysfunction Asthma, COPD, ILD Cardiomyopathy, R-to-L shunt Pulmonary Hypertension AVM Anemia, Anxiety, Obesity, Deconditioning, Hyperthyroidism, Connective Tissue Disease Obstructive PFTs yes Asthma, COPD no ILD or emphysema/ILD HRCT scan Not diagnostic Surgical Lung Biopsy Typical & consistent Confident diagnosis

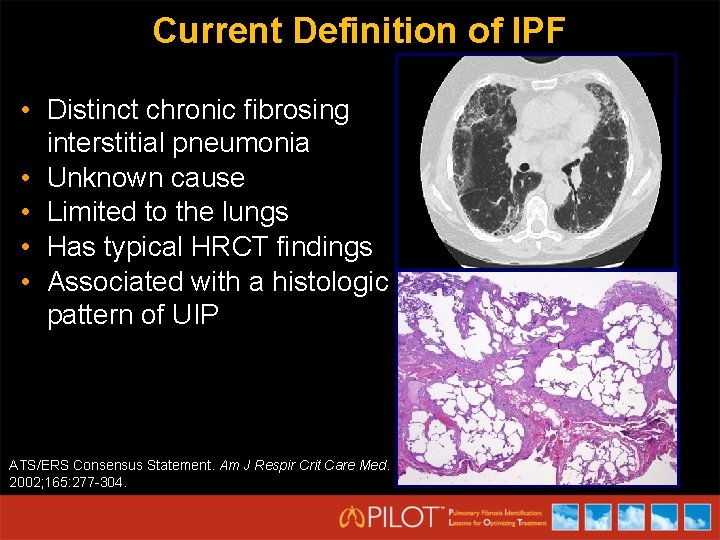
Current Definition of IPF • Distinct chronic fibrosing interstitial pneumonia • Unknown cause • Limited to the lungs • Has typical HRCT findings • Associated with a histologic pattern of UIP ATS/ERS Consensus Statement. Am J Respir Crit Care Med. 2002; 165: 277 -304.

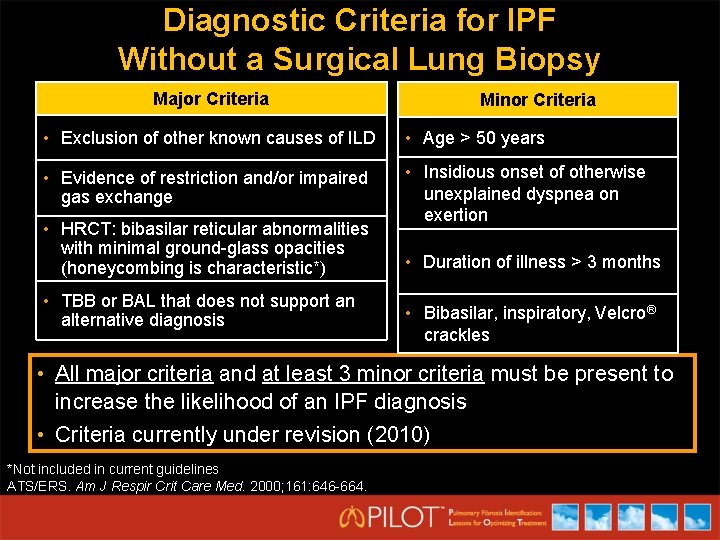
Diagnostic Criteria for IPF Without a Surgical Lung Biopsy Major Criteria Minor Criteria • Exclusion of other known causes of ILD • Age > 50 years • Evidence of restriction and/or impaired gas exchange • Insidious onset of otherwise unexplained dyspnea on exertion • HRCT: bibasilar reticular abnormalities with minimal ground-glass opacities (honeycombing is characteristic*) • TBB or BAL that does not support an alternative diagnosis • Duration of illness > 3 months • Bibasilar, inspiratory, Velcro® crackles • All major criteria and at least 3 minor criteria must be present to increase the likelihood of an IPF diagnosis • Criteria currently under revision (2010) *Not included in current guidelines ATS/ERS. Am J Respir Crit Care Med. 2000; 161: 646 -664.

Pulmonary Function Tests • Normal PFTs or resting ABG do not exclude IPF • Restriction (not always seen) – Reduced FVC and TLC – Normal or increased FEV 1/FVC ratio • Impaired gas exchange – Decreased DLCO, Pa. O 2 – Increased P(A-a)O 2 gradient – Desaturation on exercise oximetry ATS/ERS. Am J Respir Crit Care Med. 2000; 161: 646 -664.

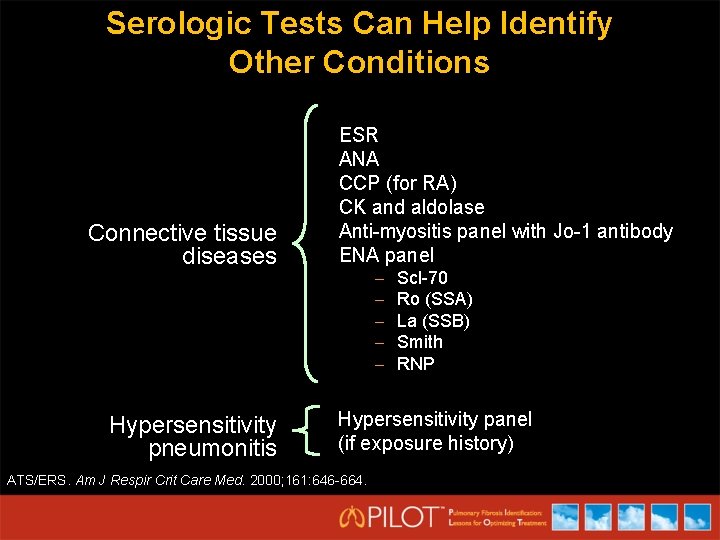
Serologic Tests Can Help Identify Other Conditions Connective tissue diseases Hypersensitivity pneumonitis ESR ANA CCP (for RA) CK and aldolase Anti-myositis panel with Jo-1 antibody ENA panel – – – Scl-70 Ro (SSA) La (SSB) Smith RNP Hypersensitivity panel (if exposure history) ATS/ERS. Am J Respir Crit Care Med. 2000; 161: 646 -664.

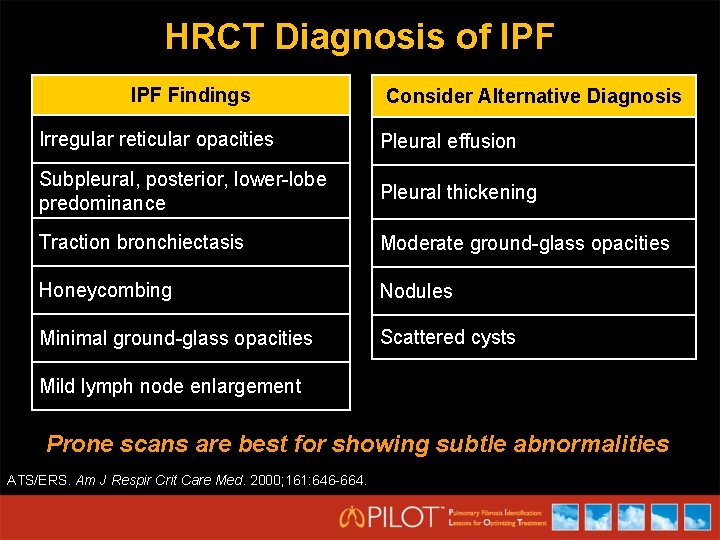
HRCT Diagnosis of IPF Findings Consider Alternative Diagnosis Irregular reticular opacities Pleural effusion Subpleural, posterior, lower-lobe predominance Pleural thickening Traction bronchiectasis Moderate ground-glass opacities Honeycombing Nodules Minimal ground-glass opacities Scattered cysts Mild lymph node enlargement Prone scans are best for showing subtle abnormalities ATS/ERS. Am J Respir Crit Care Med. 2000; 161: 646 -664.

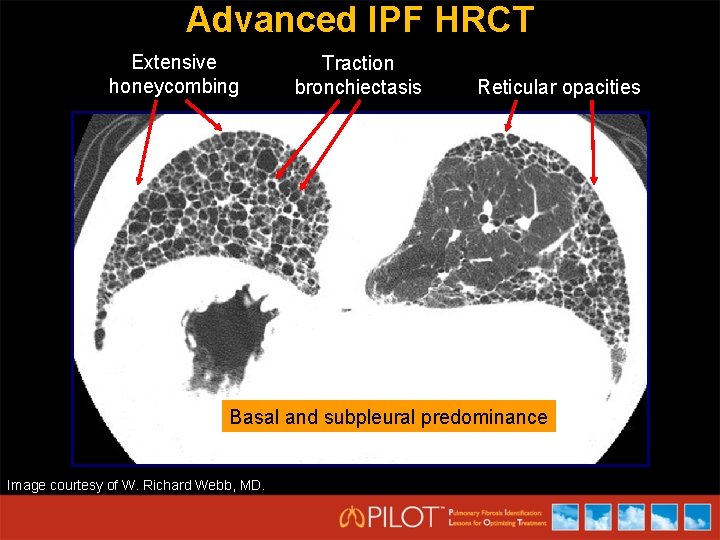
Advanced IPF HRCT Extensive honeycombing Traction bronchiectasis Reticular opacities Basal and subpleural predominance Image courtesy of W. Richard Webb, MD.

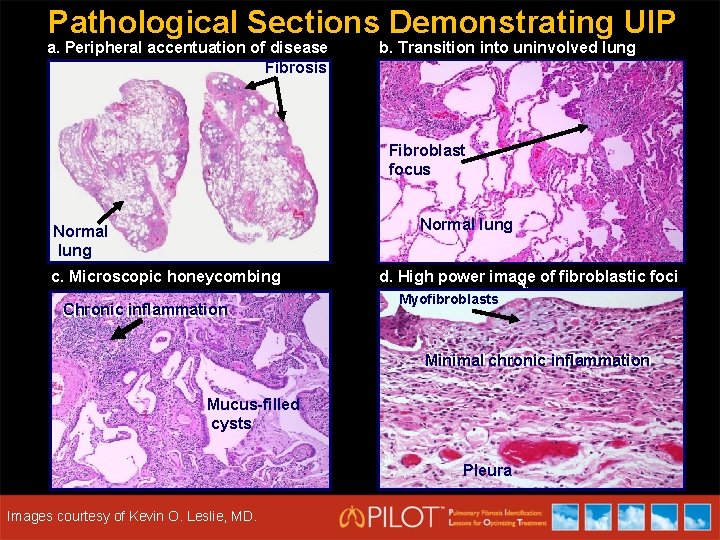
Pathological Sections Demonstrating UIP a. Peripheral accentuation of disease Fibrosis b. Transition into uninvolved lung Fibroblast focus Normal lung c. Microscopic honeycombing Chronic inflammation d. High power image of fibroblastic foci Myofibroblasts Minimal chronic inflammation Minimal Mucus-filled cysts Courtesy of Kevin O. Leslie, MD. Images courtesy of Kevin O. Leslie, MD. Pleura

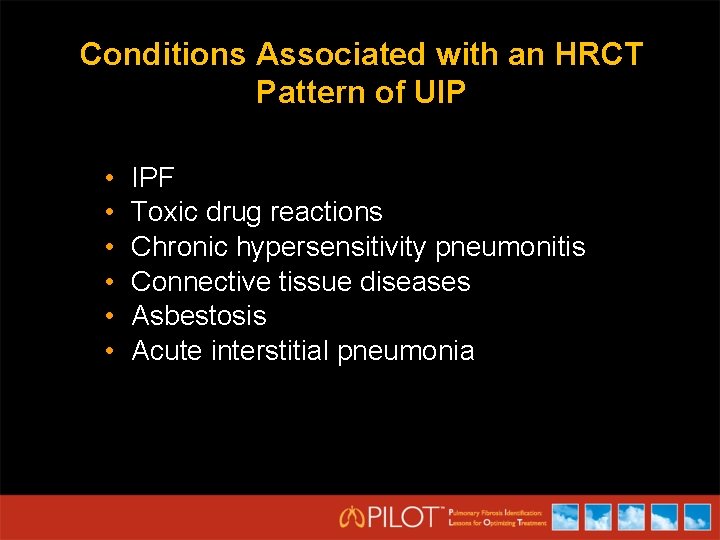
Conditions Associated with an HRCT Pattern of UIP • • • IPF Toxic drug reactions Chronic hypersensitivity pneumonitis Connective tissue diseases Asbestosis Acute interstitial pneumonia

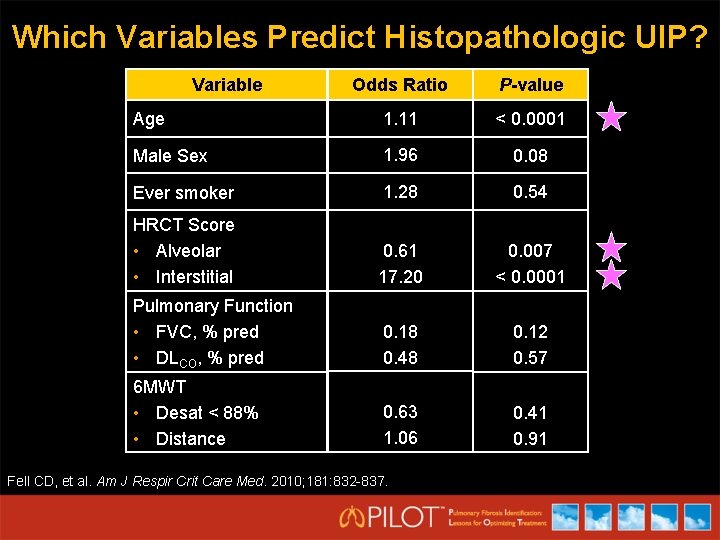
Which Variables Predict Histopathologic UIP? Variable Odds Ratio P-value Age 1. 11 < 0. 0001 Male Sex 1. 96 0. 08 Ever smoker 1. 28 0. 54 HRCT Score • Alveolar • Interstitial 0. 61 17. 20 0. 007 < 0. 0001 Pulmonary Function • FVC, % pred • DLCO, % pred 0. 18 0. 48 0. 12 0. 57 6 MWT • Desat < 88% • Distance 0. 63 1. 06 0. 41 0. 91 Fell CD, et al. Am J Respir Crit Care Med. 2010; 181: 832 -837.

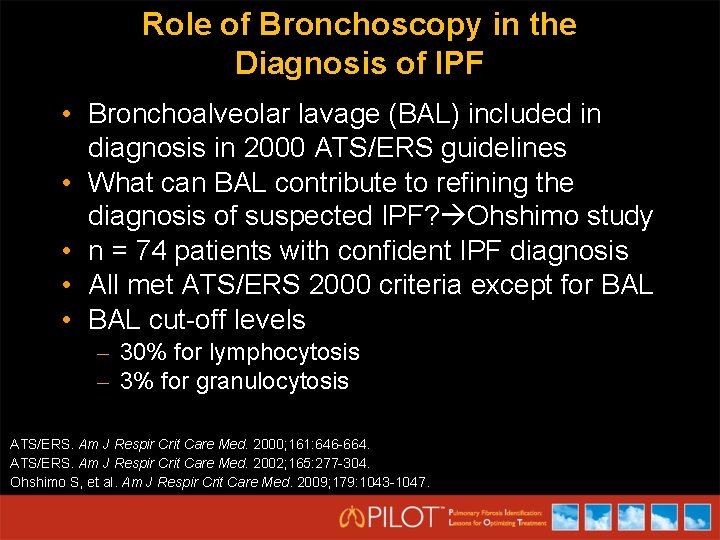
Role of Bronchoscopy in the Diagnosis of IPF • Bronchoalveolar lavage (BAL) included in diagnosis in 2000 ATS/ERS guidelines • What can BAL contribute to refining the diagnosis of suspected IPF? Ohshimo study • n = 74 patients with confident IPF diagnosis • All met ATS/ERS 2000 criteria except for BAL • BAL cut-off levels – 30% for lymphocytosis – 3% for granulocytosis ATS/ERS. Am J Respir Crit Care Med. 2000; 161: 646 -664. ATS/ERS. Am J Respir Crit Care Med. 2002; 165: 277 -304. Ohshimo S, et al. Am J Respir Crit Care Med. 2009; 179: 1043 -1047.

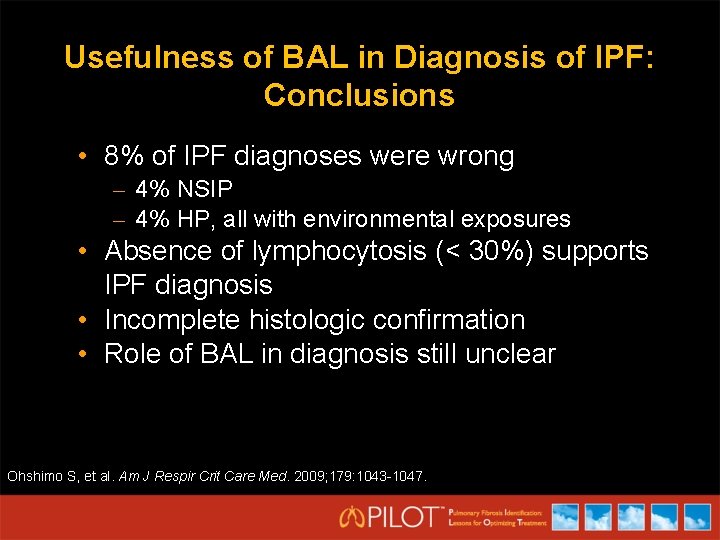
Usefulness of BAL in Diagnosis of IPF: Conclusions • 8% of IPF diagnoses were wrong – 4% NSIP – 4% HP, all with environmental exposures • Absence of lymphocytosis (< 30%) supports IPF diagnosis • Incomplete histologic confirmation • Role of BAL in diagnosis still unclear Ohshimo S, et al. Am J Respir Crit Care Med. 2009; 179: 1043 -1047.

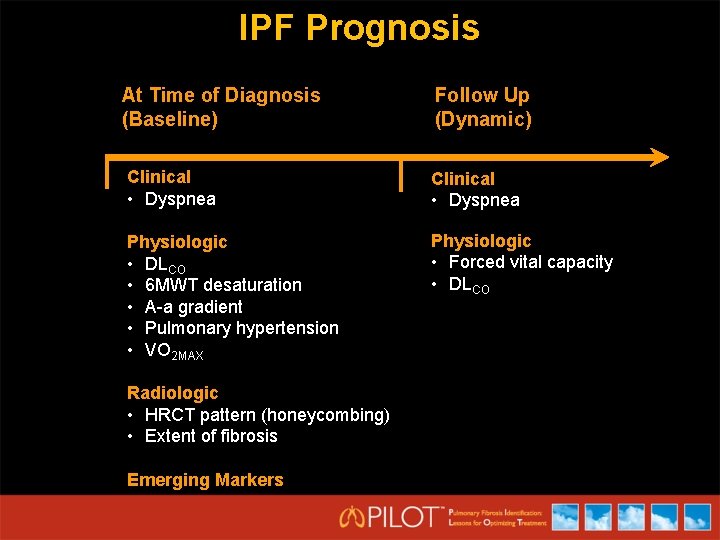
IPF Prognosis At Time of Diagnosis (Baseline) Follow Up (Dynamic) Clinical • Dyspnea Physiologic • DLCO • 6 MWT desaturation • A-a gradient • Pulmonary hypertension • VO 2 MAX Physiologic • Forced vital capacity • DLCO Radiologic • HRCT pattern (honeycombing) • Extent of fibrosis Emerging Markers

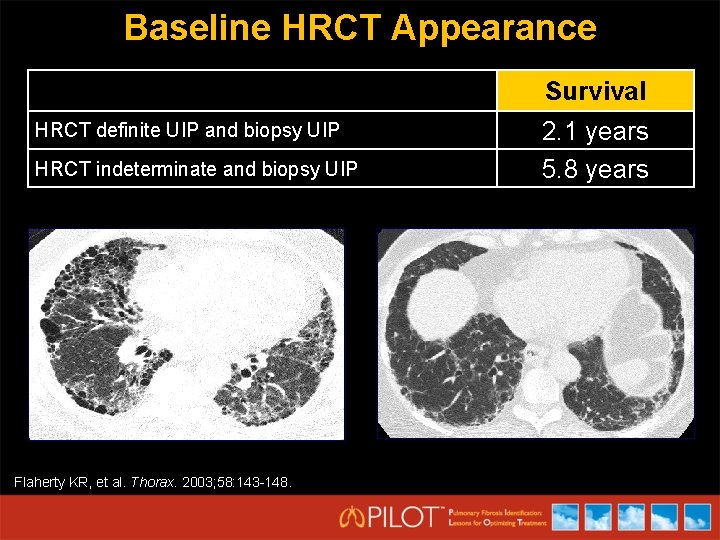
Baseline HRCT Appearance Survival HRCT definite UIP and biopsy UIP HRCT indeterminate and biopsy UIP Flaherty KR, et al. Thorax. 2003; 58: 143 -148. 2. 1 years 5. 8 years

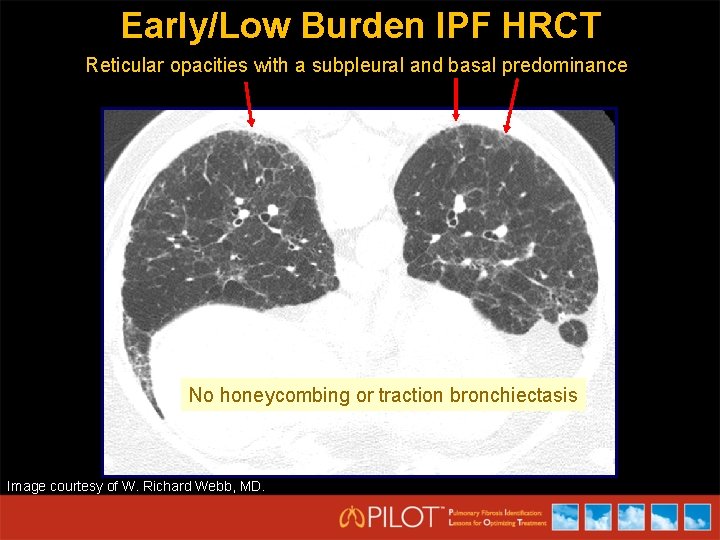
Early/Low Burden IPF HRCT Reticular opacities with a subpleural and basal predominance No honeycombing or traction bronchiectasis Image courtesy of W. Richard Webb, MD.

Baseline HRCT Findings • Sumikawa et al – 98 cases of IPF – Quantified various HRCT findings – Traction bronchiectasis and “fibrosis score” associated with survival • Best et al – 167 cases of IPF (Interferon beta trial) – Quantified various HRCT findings – Extent of fibrosis (combined reticulation and honeycombing) associated with survival Sumikawa H, et al. Am J Respir Crit Care Med. 2008; 177: 433 -439. Best AC, et al. Radiology. 2008; 246: 935 -940.

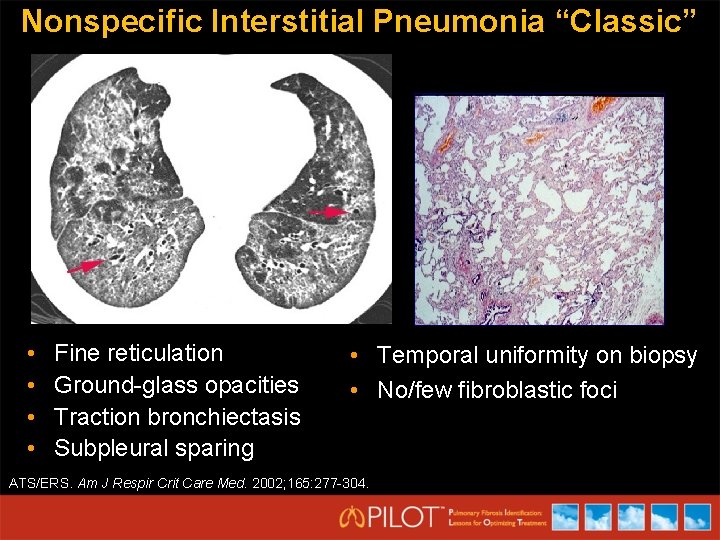
Nonspecific Interstitial Pneumonia “Classic” • • Fine reticulation Ground-glass opacities Traction bronchiectasis Subpleural sparing • Temporal uniformity on biopsy • No/few fibroblastic foci ATS/ERS. Am J Respir Crit Care Med. 2002; 165: 277 -304.

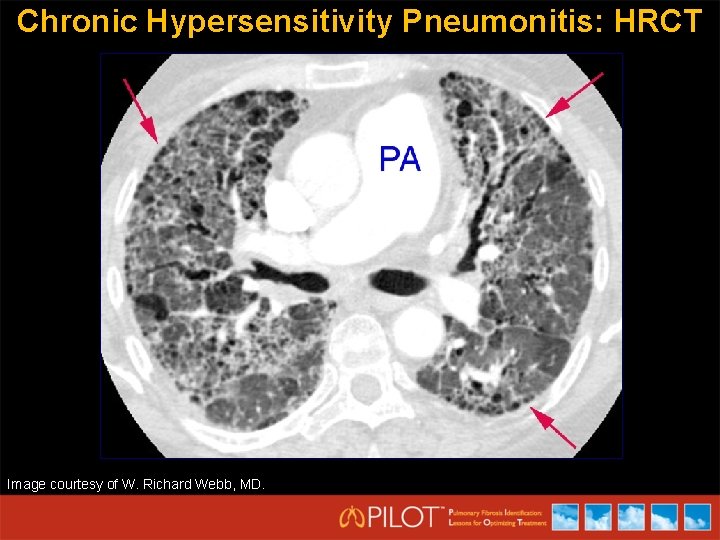
Chronic Hypersensitivity Pneumonitis: HRCT Image courtesy of W. Richard Webb, MD.

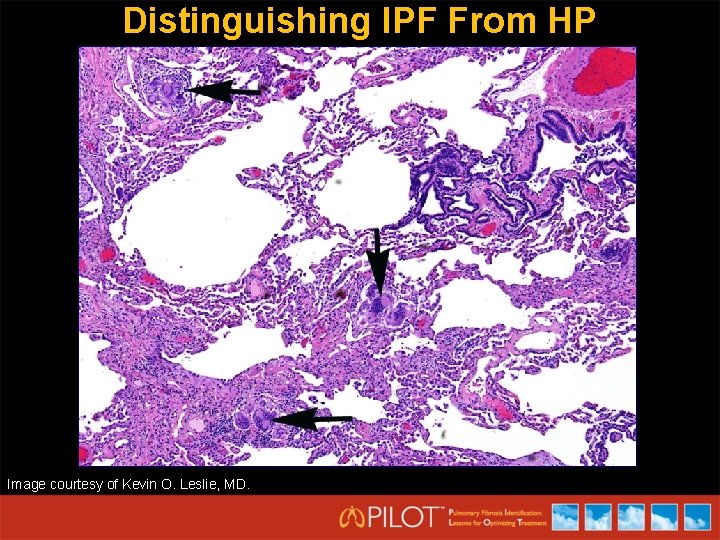
Distinguishing IPF From HP Image courtesy of Kevin O. Leslie, MD.

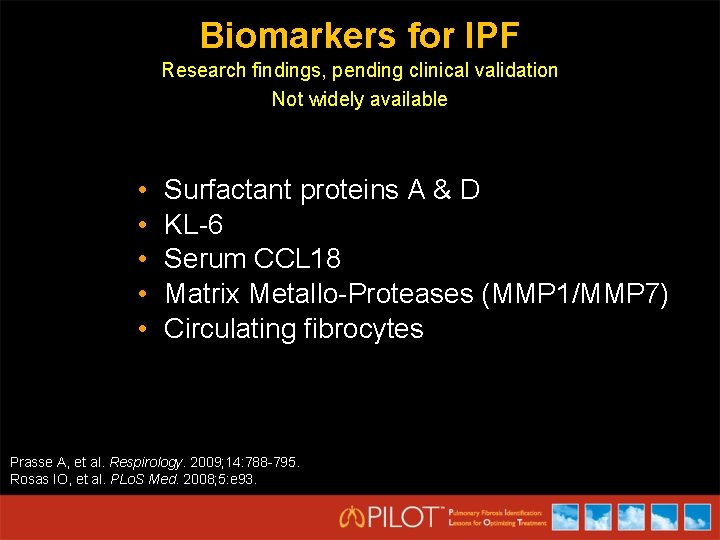
Biomarkers for IPF Research findings, pending clinical validation Not widely available • • • Surfactant proteins A & D KL-6 Serum CCL 18 Matrix Metallo-Proteases (MMP 1/MMP 7) Circulating fibrocytes Prasse A, et al. Respirology. 2009; 14: 788 -795. Rosas IO, et al. PLo. S Med. 2008; 5: e 93.

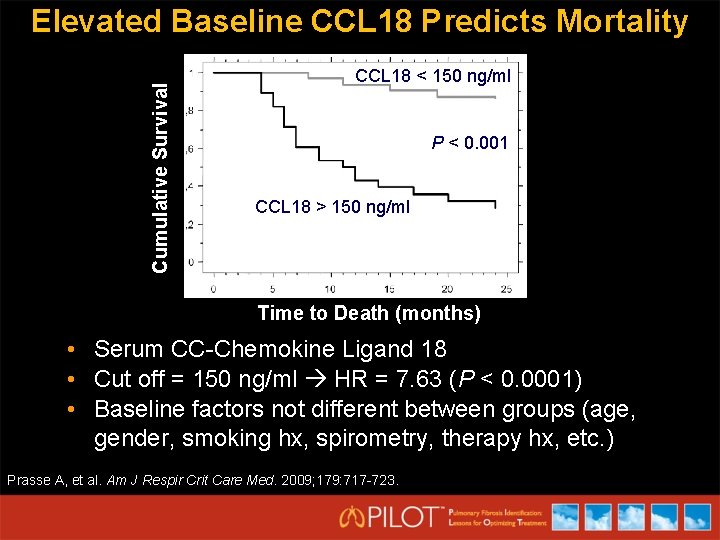
Cumulative Survival Elevated Baseline CCL 18 Predicts Mortality CCL 18 < 150 ng/ml P < 0. 001 CCL 18 > 150 ng/ml Time to Death (months) • Serum CC-Chemokine Ligand 18 • Cut off = 150 ng/ml HR = 7. 63 (P < 0. 0001) • Baseline factors not different between groups (age, gender, smoking hx, spirometry, therapy hx, etc. ) Prasse A, et al. Am J Respir Crit Care Med. 2009; 179: 717 -723.

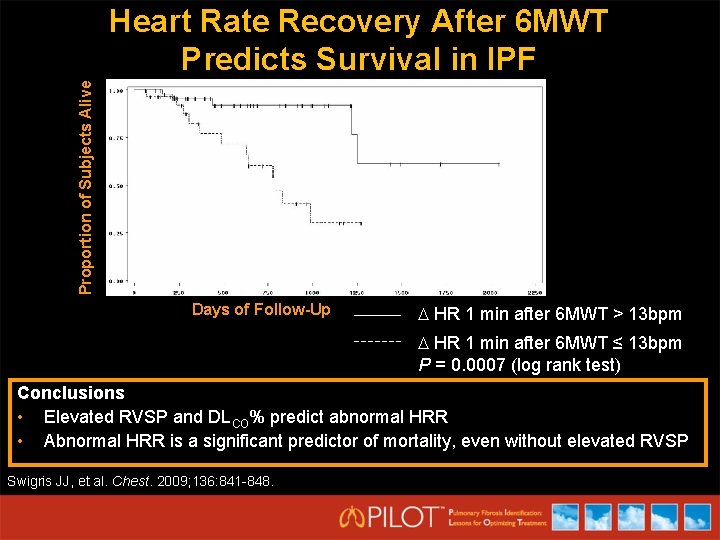
Proportion of Subjects Alive Heart Rate Recovery After 6 MWT Predicts Survival in IPF Days of Follow-Up HR 1 min after 6 MWT > 13 bpm HR 1 min after 6 MWT ≤ 13 bpm P = 0. 0007 (log rank test) Conclusions • Elevated RVSP and DLCO% predict abnormal HRR • Abnormal HRR is a significant predictor of mortality, even without elevated RVSP Swigris JJ, et al. Chest. 2009; 136: 841 -848.

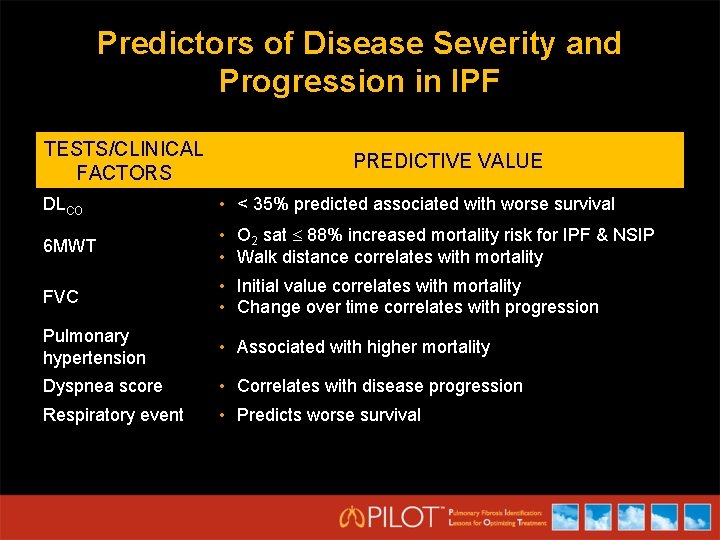
Predictors of Disease Severity and Progression in IPF TESTS/CLINICAL FACTORS PREDICTIVE VALUE DLCO • < 35% predicted associated with worse survival 6 MWT • O 2 sat 88% increased mortality risk for IPF & NSIP • Walk distance correlates with mortality FVC • Initial value correlates with mortality • Change over time correlates with progression Pulmonary hypertension • Associated with higher mortality Dyspnea score • Correlates with disease progression Respiratory event • Predicts worse survival

Monitoring Patients with IPF • Every 3 to 6 months: – – – Spirometry Diffusion 6 MWT QOL (patient questionnaire to assess dyspnea) O 2 requirement Comorbidities • HRCT should be done only with clinical change

Evaluation for Lung Transplantation • Early referral • Sp. O 2 ≤ 88%1 – Defines desaturation on 6 MWT – Calls for O 2 supplemented 6 MWT – Distinguishes early/advanced disease • Lung Allocation Score (LAS) used to prioritize candidates 2 – Survival on list (urgency) – Survival posttransplant (benefit) 1. 2. Lama VN, Flaherty KR, Toews GB, et al. Prognostic value of desaturation during a 6 -minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003; 168: 1084 -1090. A Guide to Calculating the Lung Allocation Score. http: //www. unos. org/Shared. Content. Documents/lung_allocation_score_updated_01072009. pdf. Accessed August 2010.

Diagnosis Take Home Messages • Typical clinical features include age > 50 years, gender (male > female), insidious onset of dyspnea, nonproductive cough, and bibasilar Velcro® crackles • Atypical clinical or HRCT findings in a patient with suspected IPF should prompt consideration of a surgical lung biopsy. With a confident clinical and HRCT diagnosis, a biopsy is not recommended • IPF has characteristic UIP histopathologic features that enable definitive diagnosis when clinical and HRCT findings are inconclusive

Monitoring Take Home Messages • Both baseline and dynamic factors measured during monitoring of IPF are associated with an increased risk of mortality • Prognostic indicators do not fully predict the course of disease for an individual patient • Patients should be monitored every 3 to 6 months for comorbidities and progression of IPF