Assessing Fluid Responsiveness in Critically Ill Patients Justin




















- Slides: 39

Assessing Fluid Responsiveness in Critically Ill Patients Justin Hourmozdi MD Henry Ford Hospital, Department of Emergency Medicine, EM 2. 5 S

Objectives • Review the clinical significance and literature on the topic • Specifically review the literature regarding common modalities used in the ED and ICU to assess fluid responsiveness including: physical exam, CVP, IVC ultrasound, and pulse contour analysis (SVV/PPV), EDM

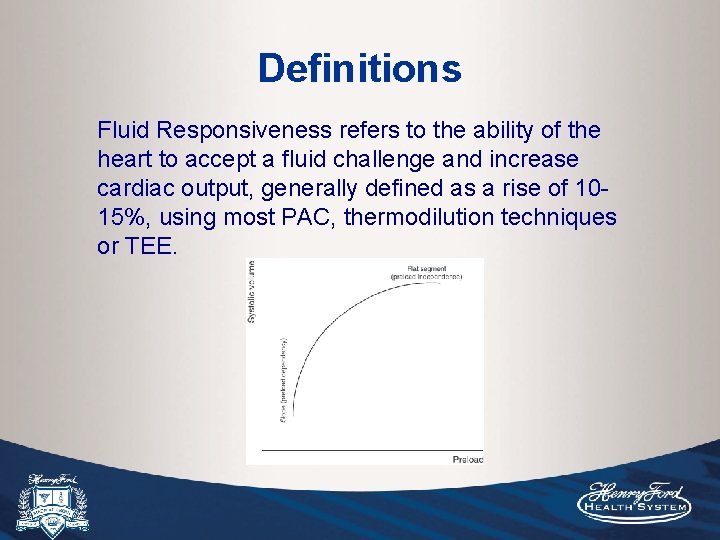
Definitions Fluid Responsiveness refers to the ability of the heart to accept a fluid challenge and increase cardiac output, generally defined as a rise of 1015%, using most PAC, thermodilution techniques or TEE.

Is my patient fluid responsive? 79 year old male who presents to the ED in septic shock with pneumonia. You have intubated the patient, placed a CVL and a-line, given 4 L of IVF to achieve a CVP of 10 -12, and started Levophed to maintain MAP. Sv. O 2 is 68%. There are no MICU beds, and you are now caring for the patient for 8 -9 hours and have to sign out to the oncoming team. Throughout your shift, the patient is having escalating Levophed requirements, O 2/PEEP requirements and plateau pressures. The patient is in oliguric renal failure. Should you give more volume, keep increasing the Levophed?

Is my patient fluid responsive? 54 year old female admitted directly to GPU from clinic with “sepsis” of unknown origin. The patient is spiking low-grade fevers and tachycardic with low UOP and rising Cr. The GPU team gives several liters of IVFs over the next 24 -36 hours empirically. The patient has increased oxygen requirements and goes into hypertensive emergency and respiratory distress and gets intubated in the MICU. You line the patient up and she has a CVP of 12 -14 and is becoming hypotensive requiring escalating doses of Levophed over the next 12 -24 hrs. She is in oliguric renal failure and your urine lytes indicate she is pre-renal. Her vent requirements are increasing to PEEP of 18, CXR is “fluffy”. Volume challenge? Increase pressors? Dialysis?

Is my patient fluid responsive? 48 year old male with a history of uncontrolled hypertension comes to the ED with a 2 day history of N/V and unable to take his meds. He is severely hypertensive. You give him all his home meds and several doses of IV antihypertensives and get an ICU bed quickly. The patient is still hypertensive and a Nicardipine drip is started and maxed out, probably when his oral meds start to have peak effect. The patient goes into shock, gets intubated, and is in anuric renal failure. His CVP is 14 -16, CXR showed mild pulmonary edema, his lower extremities have 1+ pitting edema, and he’s on high doses of Levophed with a lactate of 16. You receive the patient with instructions to call Nephrology to remove volume. His Vigeleo shows a CI of 1. 6 and SVV of 18.

Conclusions up front • Over-resuscitation with fluids leads to increased morbidity and mortality in critically ill patients. • CVP is a practical and valid tool during the initial phase of resuscitation (612 hours) to assess volume tolerance and to “fill the tank” before starting vasopressors. SSG state best evidence is to achieve filling CVP of 8 -12 within first 6 hours, however there is no clear evidence on when to discontinue or reduce fluid resuscitation after that point. • After this initial phase of resuscitation, ideally additional modalities should be used in conjunction with CVP to assess whether further fluid resuscitation is needed. IVC ultrasound and SVV/PPV are two of the more validated and practical modalities, however each have their limitations. Patients must be mechanically ventilated with TV in 8 -10 ml/kg range. Additionally for SSV/PPV analysis, patients must be in NSR, no significant cardiac disease, and sedated and synchronous with the ventilator.

Fluid balance and Prognosis Unguided large-volume resuscitation has been shown to increase extravascular lung water and resultant increased time on MV, increased ICU LOS and mortality. Also there is some evidence suggesting an increase in AFF and RRT. Survey of critically ill patients with sepsis, positive fluid balance was associated with increased mortality. Vincent JL, Sakr Y, Sprung CL, et al: Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med 2006 Positive fluid balance increased time on ventilator and trend towards increased mortality in critically ill patients with ALI. Wiedemann HP, Wheeler AP, Bernard GR, et al: Comparison of two fluid management strategies in acute lung injury. N Engl J Med 2006 Systematic review of all RCT of goal-directed fluid resuscitation reporting renal outcomes during perioperative care. In 24 perioperative studies, GD-FR was associated with decreased risk of postoperative AKI (OR 0. 59, 95% CI 0. 39 -0. 89). Prowle JR, Chua HR, Bagshaw SM, et al: Clinical review: Volume of fluid resuscitation and the incidence of acute kidney injury – a systematic review. Crit Care 2012 Examining the PROCCES data, protocol-based standard-therapy group received on average more IVFs and had higher incidence of ARF needing RRT than in the EGDT and usual care groups (6% vs 3%).

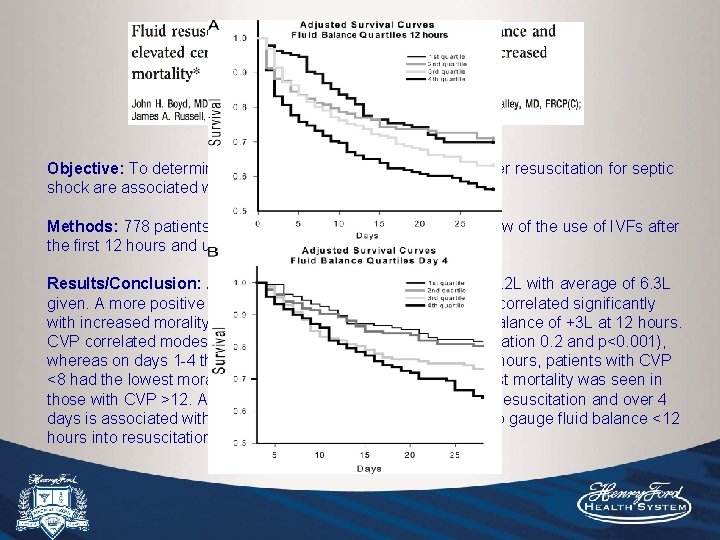
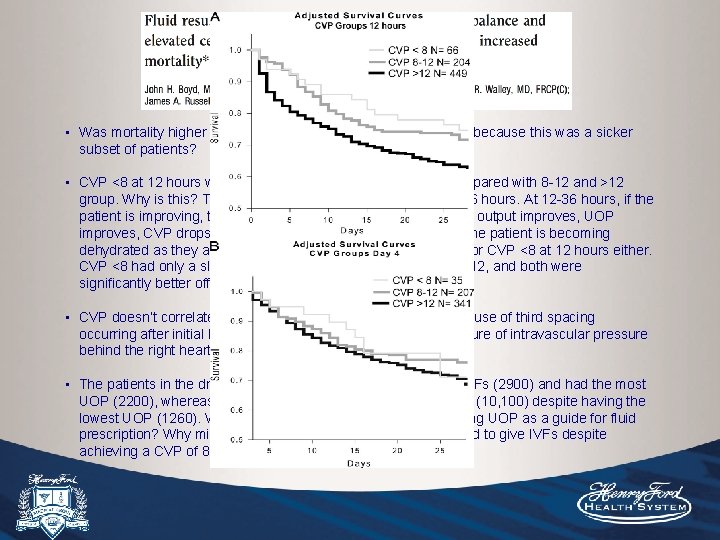
Objective: To determine whether CVP and net fluid balance after resuscitation for septic shock are associated with mortality. Methods: 778 patients from multiple centers, retrospective review of the use of IVFs after the first 12 hours and up to 4 days. Results/Conclusion: A more positive fluid balance at both 12 hours and day 4 correlated significantly with increased morality. Highest survival was seen with a fluid balance of +3 L at 12 hours. CVP correlated modestly with fluid balance at 12 hours (R correlation 0. 2 and p<0. 001), whereas on days 1 -4 there was no significant correlation. At 12 hours, patients with CVP <8 had the lowest morality rate, followed by 8 -12, and the highest mortality was seen in those with CVP >12. A more positive fluid balance both early in resuscitation and over 4 days is associated with increased mortality. CVP may be used to gauge fluid balance <12 hours into resuscitation.

Objective: To determine whether CVP and net fluid balance after resuscitation for septic shock are associated with mortality. Methods: 778 patients from multiple centers, retrospective review of the use of IVFs after the first 12 hours and up to 4 days. Results/Conclusion: Average fluid balance at 12 hours was +4. 2 L with average of 6. 3 L given. A more positive fluid balance at both 12 hours and day 4 correlated significantly with increased morality. Highest survival was seen with a fluid balance of +3 L at 12 hours. CVP correlated modestly with fluid balance at 12 hours (R correlation 0. 2 and p<0. 001), whereas on days 1 -4 there was no significant correlation. At 12 hours, patients with CVP <8 had the lowest morality rate, followed by 8 -12, and the highest mortality was seen in those with CVP >12. A more positive fluid balance both early in resuscitation and over 4 days is associated with increased mortality. CVP may be used to gauge fluid balance <12 hours into resuscitation.

• Was mortality higher in the higher fluid balance and CVP group because this was a sicker subset of patients? • “CVP <8 at 12 hours was associated with improved survival compared with 8 -12 and >12 groups. ” Why is this? This study did not look at CVP at time 0, 3, 6 hours. At 12 -36 hours, if the patient is improving, their hemodynamic state improves, CO and UOP improves, CVP drops, and they do better. This does not mean the patient is becoming dehydrated as they are improving. This does not mean to aim for CVP <8 at 12 hours either. • CVP doesn’t correlate well with fluid balance later on likely because of third spacing occurring after initial hours of fluid resuscitation, CVP is a measure of intravascular pressure behind the right heart, not total body volume. • The patients in the driest quartile received the least average IVFs (2900) and had the most UOP (2200), whereas the wettest group received the most IVFs (10, 100) despite having the lowest UOP (1260). Where these trends due to the clinician using UOP as a guide for fluid prescription? Why might have the majority of clinicians continued to give IVFs despite achieving a CVP of 8 -12?

• Was mortality higher in the higher fluid balance and CVP group because this was a sicker subset of patients? • CVP <8 at 12 hours was associated with improved survival compared with 8 -12 and >12 group. Why is this? This study did not look at CVP at time 0, 3, 6 hours. At 12 -36 hours, if the patient is improving, their hemodynamic state improves, cardiac output improves, UOP improves, CVP drops, and they do better. This does not mean the patient is becoming dehydrated as they are improving. This does not mean to aim for CVP <8 at 12 hours either. CVP <8 had only a slight mortality advantage compared with 8 -12, and both were significantly better off than >12. • CVP doesn’t correlate well with fluid balance later on likely because of third spacing occurring after initial hours of fluid resuscitation, CVP is a measure of intravascular pressure behind the right heart, not total body volume. • The patients in the driest quartiles received the least average IVFs (2900) and had the most UOP (2200), whereas the wettest group received the most IVFs (10, 100) despite having the lowest UOP (1260). Where these trends due to the clinician using UOP as a guide for fluid prescription? Why might have the majority of clinicians continued to give IVFs despite achieving a CVP of 8 -12?

Objective: Hypothesized that there is an association between quicker, adequate fluid resuscitation and patient outcome from sepsis onset time. Methods: 594 patients, retrospective review. Adjusted for total fluid administration within the first 6 hours of sepsis onset, additionally adjusted for age and severity scores. Outcome was in-hospital mortality. Results: Median amount of fluid within first 3 hours for survivors was 2, 085 m. L (9404, 080) vs non-survivors 1, 600 m. L (600 -3, 010) [p=0. 007]. In comparison, during the 3 -6 hours, 660 m. L vs 800 m. L (p=0. 09). The higher proportion of total fluid received within the first 3 hours was associated with decreased hospital mortality (OR 0. 34, 95% CI 0. 15 -0. 75, p=0. 008).

Objective: To determine the role of fluid resuscitation in the treatment of children with septic shock and life-threatening infections. Most children in hospitals in sub. Saharan Africa receive no specific fluid management apart from blood transfusion for severe anemia or MIVFs. They wanted to compare international standard of practice (bolus-fluid resuscitation) to local standard of care (no bolus-fluid resuscitation). Methods: MC-RCT of 3, 141 children in Africa. 3 groups: 5% Albumin bolus, NS bolus, no bolus for children with severe febrile illness and impaired perfusion (AMS, resp distress, cap refill >3 s, cool exts, weak pulse, severe tachycardia, 39% lactate >5). Children with severe hypotension were randomly assigned to bolus groups only. Primary end-point was 48 -hr mortality, secondary included 28 -day mortality. Results: 48 -hr mortality was 10. 6%, 10. 5%, and 7. 3%, 28 -day mortality was 12. 2%, 12%, 8. 7% (p=0. 004 for any bolus vs control).

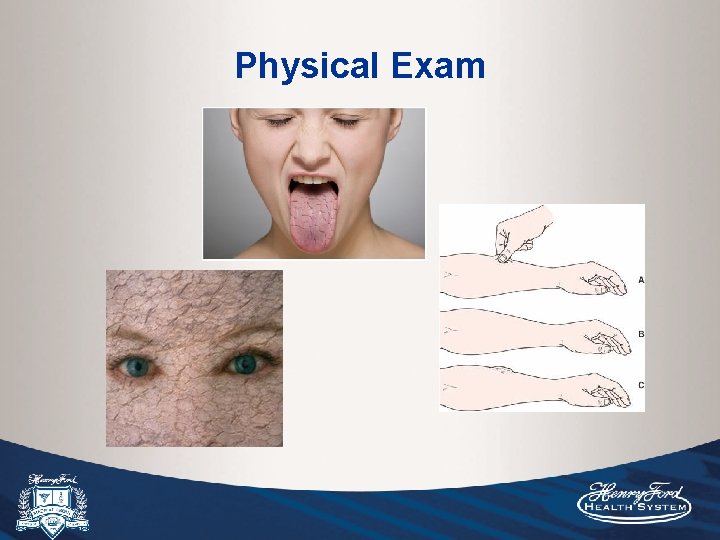
Physical Exam

• Systematic review of 10 studies investigating postural vital signs or cap refill of healthy volunteers who underwent phlebotomy. Most helpful physical findings are severe postural dizziness, postural pulse increase of >= 30 (measured 1 minute after standing). Presence of either finding has a sensitivity of only 22% for moderate blood loss (~500 m. L), but much greater sensitivity for large (~1 L) blood loss (97%) and specificity (98%). • Postural hypotension (drop in SBP >20) was not sensitive nor specific, and occurs in up to 10% or normovolemic adults <65 and 11 -30% >65. A study of 911 elderly NH patients in this review found that about 50% were orthostatic. • Four studies of patients presenting to the ED with suspected hypovolemia due to N/V/D. The presence of a dry axilla supports hypovolemia (LR+2. 8) and moist MMs and a tongue without furrows argue against it (LR-0. 3). In adults, cap refill and skin turgor have no proven diagnostic value.

One of the more recent of a number of trials showing that estimation of volume status in critically ill patients based on physical exam showed poor correlation with volume status and poor interobserver agreement. • Physical exam is unreliable in assessing volume status, especially in critically ill patients. Vital signs can be non-specific, UOP can be misleading if in ATN, peripheral edema does not always correlate with intravascular volume status, skin and mucus membrane changes are subject to interobserver variability, environmental conditions, medications (anticholinergic effects of many medications). • Just give a bolus and reassess physical exam: check RR, pulse ox and listen for crackles, but should pulmonary edema really be an end point of fluid resuscitation?

Central Venous Pressure CVP is a good approximation of RAP, which is a major determinant of RV filling. Assuming that CVP is a good indicator of RV preload and because RV SV determines LV preload, then CVP is assumed to be an indirect measure of LV preload. CVP is influenced by the patients’ vascular tone and hemodynamic status, RV and LV compliance, lung compliance, presence of tricuspid valve abnormalities, pulmonary hypertension, and intraabdominal pressure. Therefore, CVP is best interpreted in the clinical context of the patient, using other hemodynamic and metabolic end-points.

Pressure or Volume? mm. Hg 20 Normal LV with high EDV 20 Poor LV with reduced compliance and lower EDV

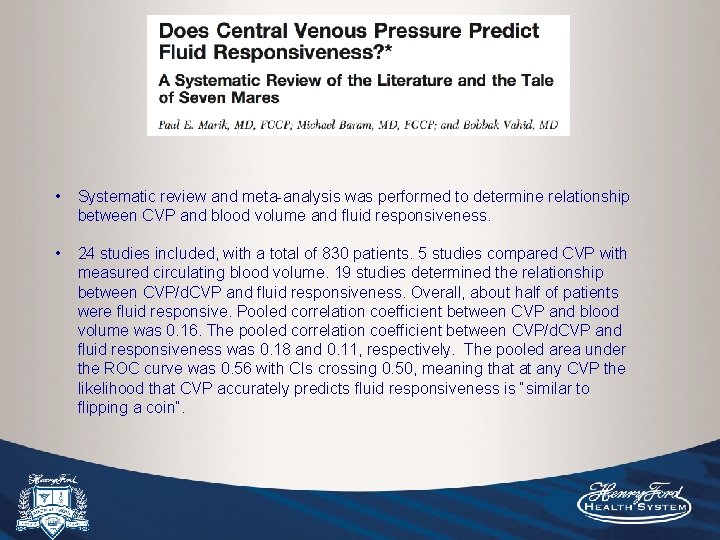
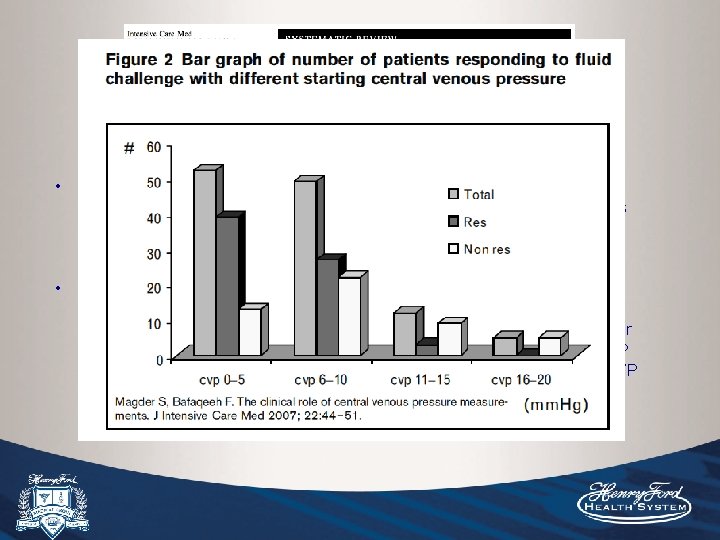
• Systematic review and meta-analysis was performed to determine relationship between CVP and blood volume and fluid responsiveness. • 24 studies included, with a total of 830 patients. 5 studies compared CVP with measured circulating blood volume. 19 studies determined the relationship between CVP/d. CVP and fluid responsiveness. Overall, about half of patients were fluid responsive. Pooled correlation coefficient between CVP and blood volume was 0. 16. The pooled correlation coefficient between CVP/d. CVP and fluid responsiveness was 0. 18 and 0. 11, respectively. The pooled area under the ROC curve was 0. 56 with CIs crossing 0. 50, meaning that at any CVP the likelihood that CVP accurately predicts fluid responsiveness is “similar to flipping a coin”.

• Baseline CVP was 8. 7+/-2. 3 in responders compared to 9. 7+/-2. 2 in nonresponders (non-significant difference). Is this a collection of mostly volume replete ICU patients, most of whom are several days into their hospital course? • True, small differences in target-range CVPs may not predict volume responsiveness, but patients who are very dry often have CVP values of <5 that will significantly increase after volume expansion. I believe there is insufficient evidence from this review to show lack of relationship for CVP during the initial phase of resuscitation, and possibly at more extreme values, such as <5 or >13 where a correlation could exist.

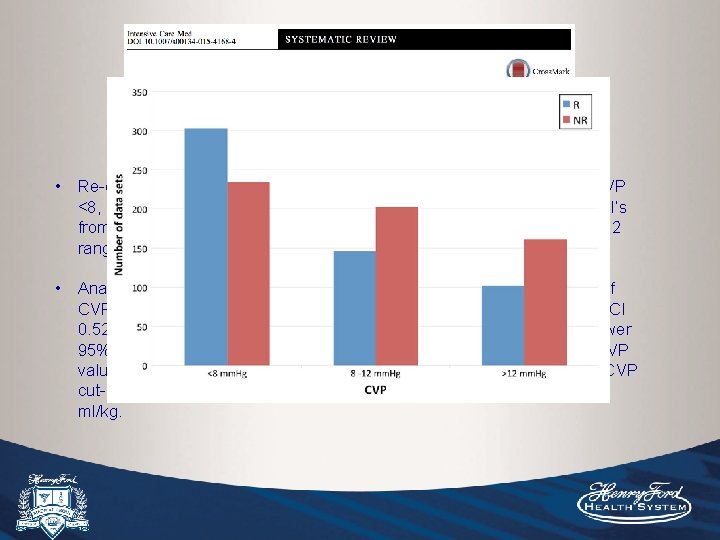
• Re-evaluated CVP for FR looking at a larger sample subgrouped by CVP <8, 8 -12, >12. 51 studies included, raw data sets were obtained from PI’s from each study, of which the majority had mean CVP values in the 8 -12 range. • 1, 148 patients allowed subgroup analysis of CVP <8, which showed area under ROC curve of 0. 57 (95% CI 0. 52 -0. 62), in contrast to the CVP 8 -12 and >12 groups in which the lower 95% CI crossed 0. 50. • Identified some modest PPV/NPV for low/high CVP values. The highest PPV was at CVP cut-off of 2 -4 (65%) and NPV at CVP cut-off of 14 -16 (66%). About 2/3 were MV with TVs ranging from 5 -12 ml/kg.

• Re-evaluated CVP for FR looking at a larger sample subgrouped by CVP <8, 8 -12, >12. 51 studies included, raw data sets were obtained from PI’s from each study, of which the majority had mean CVP values in the 8 -12 range. • Analysis of 1, 148 patients from 22 studies allowed subgroup analysis of CVP <8, which showed area under ROC curve above 0. 50 (0. 57, 95% CI 0. 52 -0. 62), in contrast to the CVP 8 -12 and >12 groups in which the lower 95% CI crossed 0. 50. Identified some modest PPV/NPV for low/high CVP values. The highest PPV was at CVP cut-off of 2 -4 (65%) and NPV at CVP cut-off of 14 -16 (66%). About 2/3 were MV with TVs ranging from 5 -12 ml/kg.

• Re-evaluated CVP for FR looking at a larger sample subgrouped by CVP <8, 8 -12, >12. 51 studies included, raw data sets were obtained from PI’s from each study, of which the majority had mean CVP values in the 8 -12 range. • Analysis of 1, 148 patients from 22 studies allowed subgroup analysis of CVP <8, which showed area under ROC curve above 0. 50 (0. 57, 95% CI 0. 52 -0. 62), in contrast to the CVP 8 -12 and >12 groups in which the lower 95% CI crossed 0. 50. Identified some modest PPV/NPV for low/high CVP values. The highest PPV was at CVP cut-off of 2 -4 (65%) and NPV at CVP cut-off of 14 -16 (66%). About 2/3 were MV with TVs ranging from 5 -12 ml/kg.

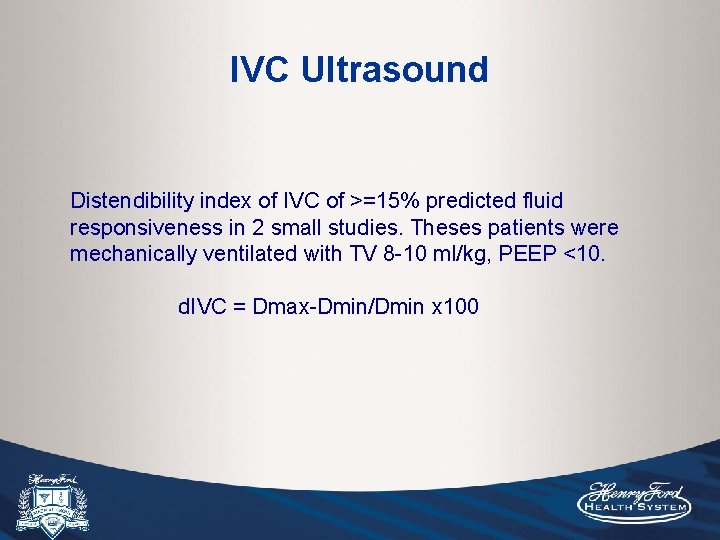
IVC Ultrasound Distendibility index of IVC of >=15% predicted fluid responsiveness in 2 small studies. Theses patients were mechanically ventilated with TV 8 -10 ml/kg, PEEP <10. d. IVC = Dmax-Dmin/Dmin x 100

IVC Ultrasound Distendibility index of IVC of >=15% predicted fluid responsiveness in 2 small studies. Theses patients were mechanically ventilated with TV 8 -10 ml/kg, PEEP <10. d. IVC = Dmax-Dmin/Dmin x 100

Methods: 23 patients with septic shock and mechanically ventilated. CI was determined by ECHO doppler of the PA trunk. CI measured before and after a 7 ml/kg bolus. Results: Using a threshold of d. IVC of 18%, responders and non-responders were discriminated with a 90% sensitive and specificity. Of note, MV parameters were TV 8. 5+/-1. 5 ml/kg, PEEP <10, Plat Pressure <30.

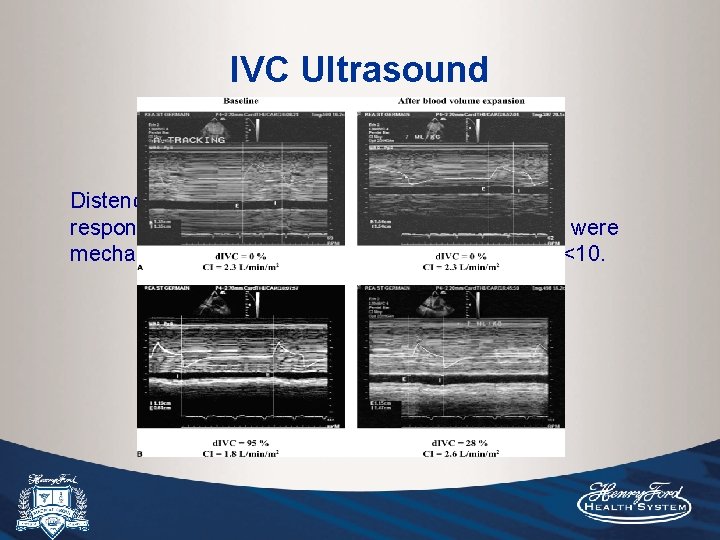
Methods: 39 patients with septic shock and MV. Patients were again ventilated with TV of 8 -10 ml/kg. CO was assessed by ECHO (measuring the diameter of the aortic orifice and the velocity time integral of aortic blood flow during end-exp before and after fluid bolus). Results: Very close relationship was observed with pre-infusion d. IVC (r=0. 82, p<0. 001), and 12% d. IVC cut-off value allowed identification of responders with PPV 93% and NPV 92%.

Objective: Determine if d. IVC is an accurate measurement of FR in non-MV ED patients. Methods: 26 non-MV patients. FR asssessed by impedance cardiography following passive leg raise. Results: d. IVC did not predict FR (receiver operating curve=0. 46, P=0. 63).

Objective: Compare the effect of ECHO-guided therapy to standard management (EGDT) following “early resuscitation for shock”, which means they were enrolled after the treating team had achieved SSG targets of CVP 812 and were then requiring pressors to maintain MAP>65. Methods: 220 septic and cardiogenic shock patients, 110 studied prospectively in ECHO group over 1 year, and 110 studied retrospectively in the year prior. All patients were MV. ECHO was performed within 24 hrs of admission. d. IVC >15% were considered volume responsive. Results: Prior to enrollment, patients had received about 65 ml/kg, achieved a CVP of >8, on Levo at ~10 mcg on average, and had cleared their lactate elevations. ECHO group during first 24 hrs had significantly lower fluid prescription (49 vs 66 ml/kg, p=0. 01), reduced stage 3 AKI (20% vs 39%), more days alive and free of RRT (28 vs 25 days), and improved 28 -day survival (66% vs 56% p=0. 04).

• This study is again looking at the sub-acute phase of shock after the initial resuscitation using end-points like CVP and MAP, and provides some evidence that this method of limiting fluids after the initial phase of resuscitation may improve outcomes. • Obvious weakness is the methodology and non-randomized nature of the study.

Pulse Contour Analysis (SVV/PPV) To use pulse contour analysis patient must have an arterial line, mechanical ventilation with TV 8 -10 ml/kg, NSR, no valvular heart disease, deeply sedated and synchronous with ventilator. In general, SVV >12% is considered to be FR.

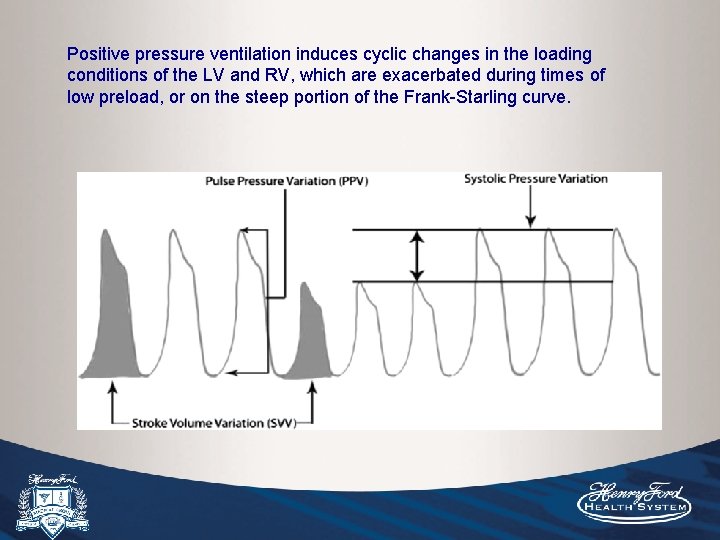
Positive pressure ventilation induces cyclic changes in the loading conditions of the LV and RV, which are exacerbated during times of low preload, or on the steep portion of the Frank-Starling curve.


• 568 critically ill patients from 23 studies. • SVV was correlated to FR with a pooled correlation coefficient of 0. 72, pooled AUC of 0. 84 and a sensitivity and specificity of about 80% for predicting FR, improved to about 85% in the ICU vs OR subgroup. • Most patients were MV with TV >8 ml/kg, although 5 studies used slightly lower TVs. A number of studies excluded patients with low EF (usually <30%) and high BMI (>40).

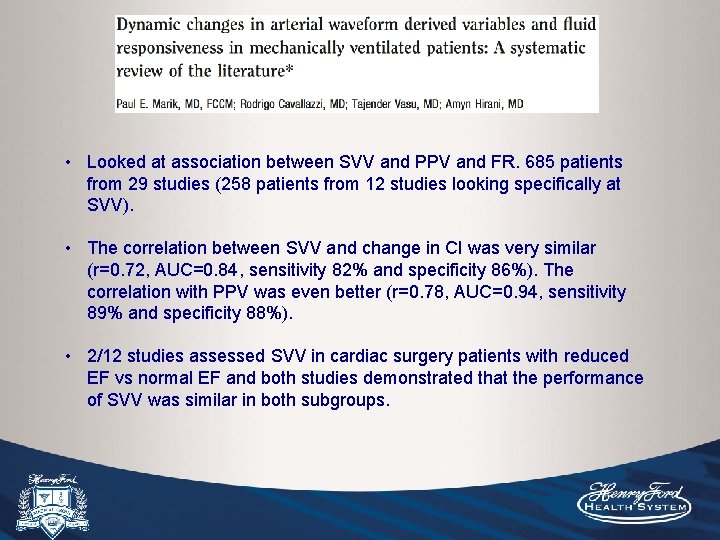
• Looked at association between SVV and PPV and FR. 685 patients from 29 studies (258 patients from 12 studies looking specifically at SVV). • The correlation between SVV and change in CI was very similar (r=0. 72, AUC=0. 84, sensitivity 82% and specificity 86%). The correlation with PPV was even better (r=0. 78, AUC=0. 94, sensitivity 89% and specificity 88%). • 2/12 studies assessed SVV in cardiac surgery patients with reduced EF vs normal EF and both studies demonstrated that the performance of SVV was similar in both subgroups.

IVC variation compared to pulse contour analysis as predictors of fluid responsiveness: a prospective cohort study. 2011. J of Int Care Med. (abstract only) Objective: To simultaneously assess ability to predict FR using SVV obtained with Vigileo and d. IVC in critically ill patients on MV. Methods: 25 MICU patients (12 ARDS, 10 sepsis, 3 cardiac arrest) undergoing MV that required vasopressors, had worsening organ function and were well adapted to the ventilator. TV was 8. 6 ml/kg +/- 1. 7. Excluded patients on hemodialysis, ascites, afib, and HR >120. Results/Conclusion: d. IVC correlated well with ROC curve of 0. 81; while SVV was 0. 57. d. IVC is superior to SVV obtained with Vigileo (possibly due to lack of calibration, unlike Pi. CCO).

Esophageal Doppler Monitor

Conclusion • Despite what the nay-sayers claim, CVP is still a practical and valid tool during the initial phase of resuscitation. • Be mindful when using CVP during that initial phase, and more importantly afterwards. After initial goals are met, ideally additional modalities should be used to guide fluid prescription, and this phase is also critical in the morbidity and mortality of your patients. However, these modalities are not without their own specific practical limitations.