ASSAY OF ASPIRIN The main methods used in




- Slides: 11

ASSAY OF ASPIRIN The main methods used in quantitative determination of ASA either in its pharmaceutical preparations or powdered pure form are: 1 - Spectrophotometric methods: . 2 - Chromatographic methods: such as: GC, TLC, and HPLC. 3 - Titration (volumetric) methods: are very simple and accurate methods; therefore, these methods are the most widely used for the quantitative determination of ASA such as:

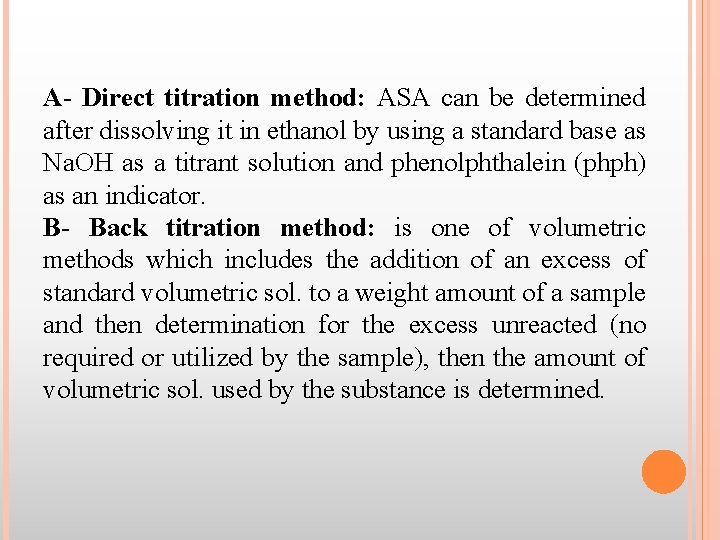
A- Direct titration method: ASA can be determined after dissolving it in ethanol by using a standard base as Na. OH as a titrant solution and phenolphthalein (phph) as an indicator. B- Back titration method: is one of volumetric methods which includes the addition of an excess of standard volumetric sol. to a weight amount of a sample and then determination for the excess unreacted (no required or utilized by the sample), then the amount of volumetric sol. used by the substance is determined.

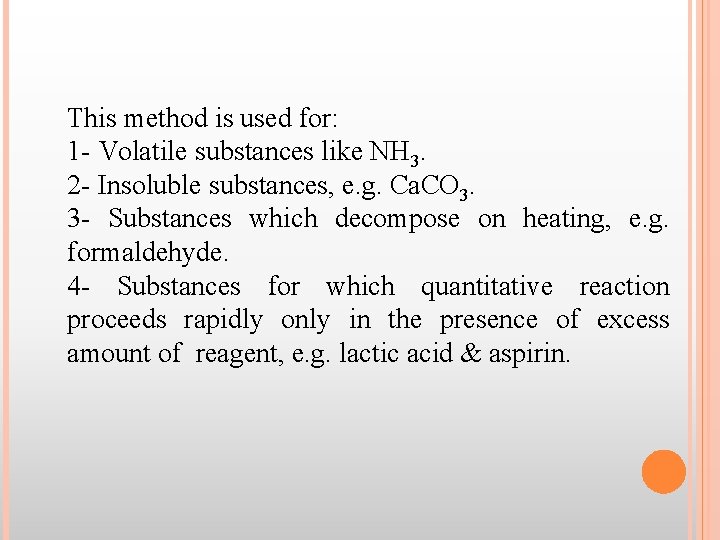
This method is used for: 1 - Volatile substances like NH 3. 2 - Insoluble substances, e. g. Ca. CO 3. 3 - Substances which decompose on heating, e. g. formaldehyde. 4 - Substances for which quantitative reaction proceeds rapidly only in the presence of excess amount of reagent, e. g. lactic acid & aspirin.

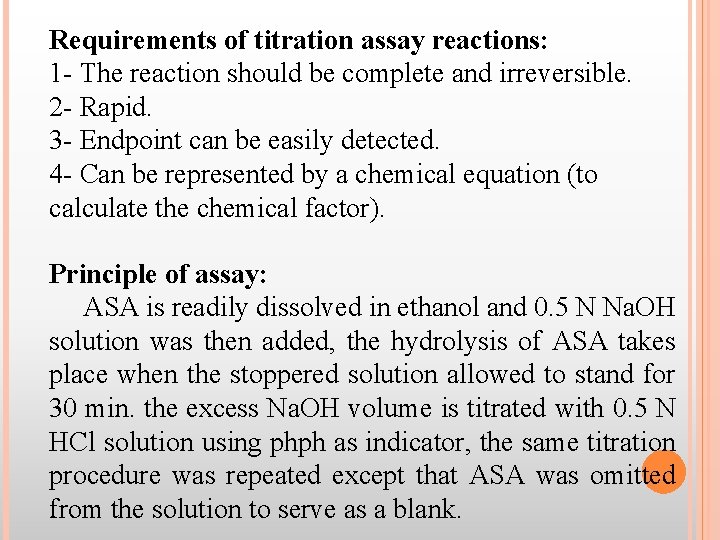
Requirements of titration assay reactions: 1 - The reaction should be complete and irreversible. 2 - Rapid. 3 - Endpoint can be easily detected. 4 - Can be represented by a chemical equation (to calculate the chemical factor). Principle of assay: ASA is readily dissolved in ethanol and 0. 5 N Na. OH solution was then added, the hydrolysis of ASA takes place when the stoppered solution allowed to stand for 30 min. the excess Na. OH volume is titrated with 0. 5 N HCl solution using phph as indicator, the same titration procedure was repeated except that ASA was omitted from the solution to serve as a blank.

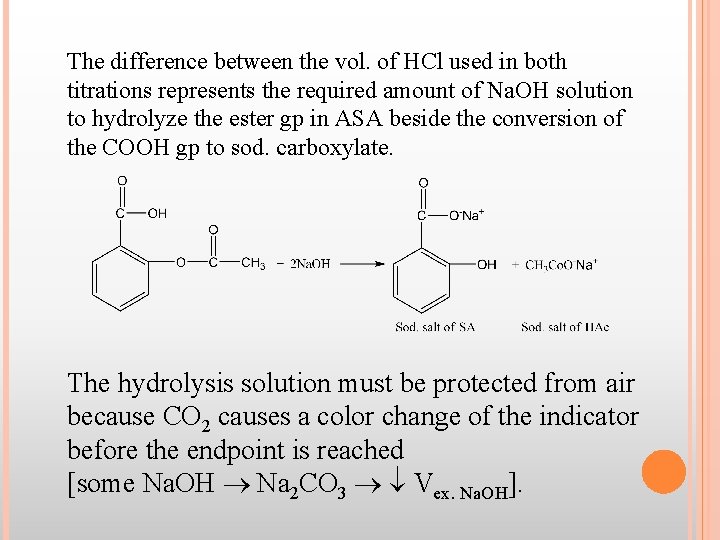
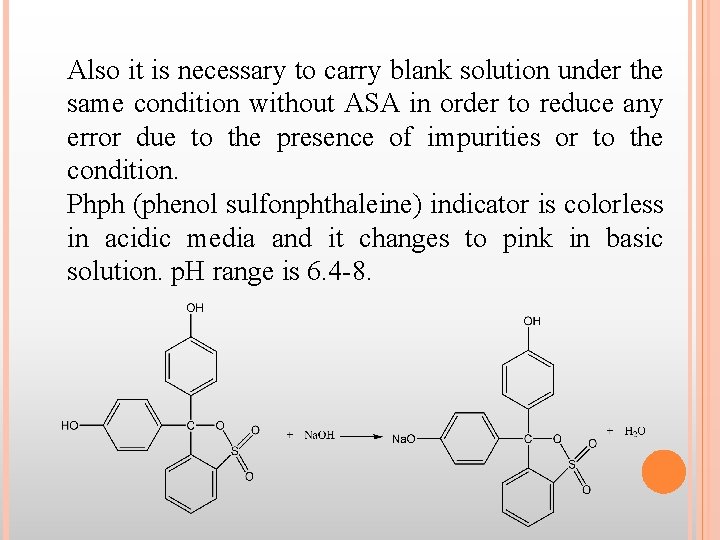
The difference between the vol. of HCl used in both titrations represents the required amount of Na. OH solution to hydrolyze the ester gp in ASA beside the conversion of the COOH gp to sod. carboxylate. The hydrolysis solution must be protected from air because CO 2 causes a color change of the indicator before the endpoint is reached [some Na. OH Na 2 CO 3 Vex. Na. OH].

Also it is necessary to carry blank solution under the same condition without ASA in order to reduce any error due to the presence of impurities or to the condition. Phph (phenol sulfonphthaleine) indicator is colorless in acidic media and it changes to pink in basic solution. p. H range is 6. 4 -8.

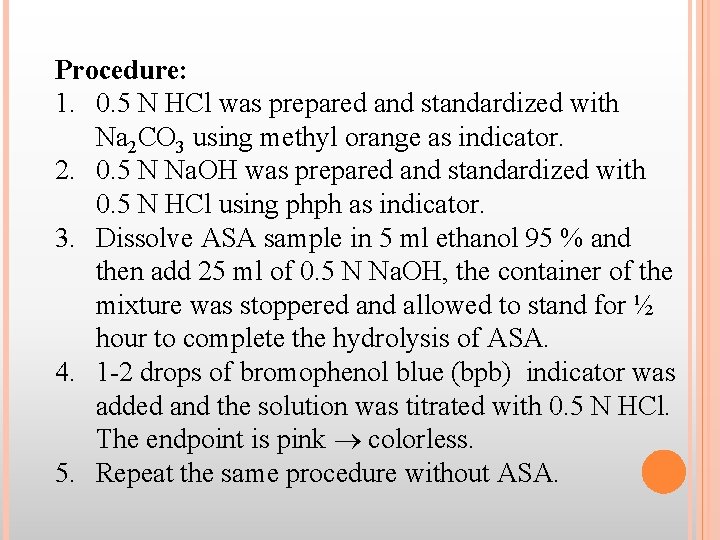
Procedure: 1. 0. 5 N HCl was prepared and standardized with Na 2 CO 3 using methyl orange as indicator. 2. 0. 5 N Na. OH was prepared and standardized with 0. 5 N HCl using phph as indicator. 3. Dissolve ASA sample in 5 ml ethanol 95 % and then add 25 ml of 0. 5 N Na. OH, the container of the mixture was stoppered and allowed to stand for ½ hour to complete the hydrolysis of ASA. 4. 1 -2 drops of bromophenol blue (bpb) indicator was added and the solution was titrated with 0. 5 N HCl. The endpoint is pink colorless. 5. Repeat the same procedure without ASA.

Note: Back titration of the excess alkali is necessary to carry out a blank experiment without acetyl salicylic acid. Heating and cooling of alkaline liquid results in an apparent changes in the strength of certain indicators used. This may be due to the absorption of atmospheric CO 2 gas. The CO 2 is rapidly absorbed by the hot sol (Na. OH) to form sod. carbonate. In the back titration , CO 2 causes a color change of indicator before the actual endpoint.

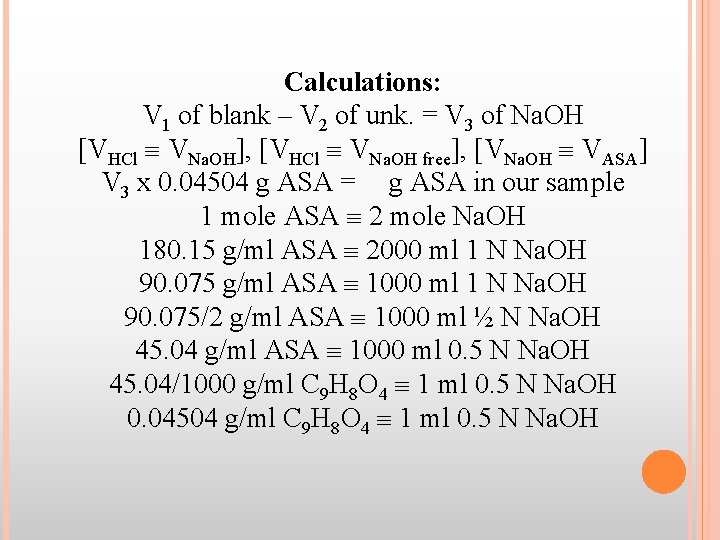
Calculations: V 1 of blank – V 2 of unk. = V 3 of Na. OH [VHCl VNa. OH], [VHCl VNa. OH free], [VNa. OH VASA] V 3 x 0. 04504 g ASA = g ASA in our sample 1 mole ASA 2 mole Na. OH 180. 15 g/ml ASA 2000 ml 1 N Na. OH 90. 075 g/ml ASA 1000 ml 1 N Na. OH 90. 075/2 g/ml ASA 1000 ml ½ N Na. OH 45. 04 g/ml ASA 1000 ml 0. 5 N Na. OH 45. 04/1000 g/ml C 9 H 8 O 4 1 ml 0. 5 N Na. OH 0. 04504 g/ml C 9 H 8 O 4 1 ml 0. 5 N Na. OH

Q 1/ why we use warm water in recrystallization of ASA? Q 2/what is the purpose of activated charcoal?

Q 1/what are the substances that could be used for back titration? Q 2/if the vol. of HCl for unk 20 ml and the vol of HCl of blank 30 ml and we use the HCl and Na. OH 2 N , what the amount of aspirin ?