Aspen Thermodynamic Models Group 3 Andrew Lum Vu









- Slides: 20

Aspen: Thermodynamic Models Group 3 Andrew Lum, Vu Pham, Sofea Rosdan, Nathan Wong, Kyle Yamaguchi, Kelly Zhang

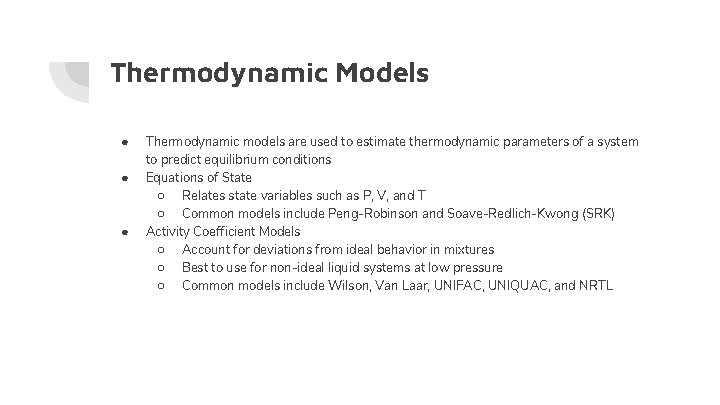
Thermodynamic Models ● ● ● Thermodynamic models are used to estimate thermodynamic parameters of a system to predict equilibrium conditions Equations of State ○ Relates state variables such as P, V, and T ○ Common models include Peng-Robinson and Soave-Redlich-Kwong (SRK) Activity Coefficient Models ○ Account for deviations from ideal behavior in mixtures ○ Best to use for non-ideal liquid systems at low pressure ○ Common models include Wilson, Van Laar, UNIFAC, UNIQUAC, and NRTL

Aspen Plus ● ● Chemical Process Simulator Contains libraries of commonly used thermodynamic models

Why You Need to Choose the Correct Model ● ● ● Enable correct calculations for ○ Pressure, temperature, composition and specific volume for multi-component and multi-phase chemical systems. Determine the state functions of a system, such as enthalpy and entropy and hence, Gibbs free energy. Incorrect models can lead to convergence failures in ASPEN due to difference in parameters ○ Will lead to misleading and incorrect results in simulation

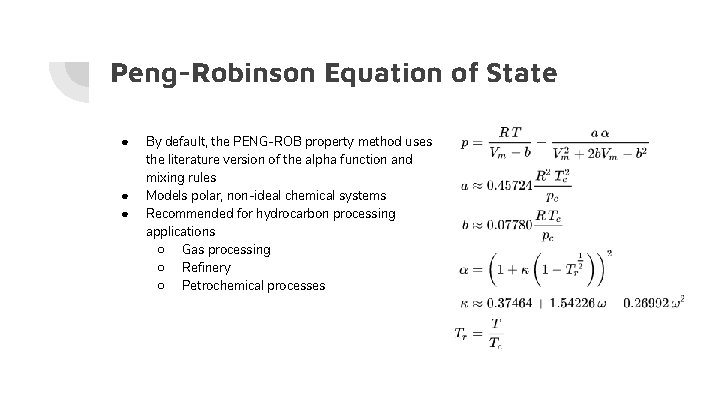
Peng-Robinson Equation of State ● ● ● By default, the PENG-ROB property method uses the literature version of the alpha function and mixing rules Models polar, non-ideal chemical systems Recommended for hydrocarbon processing applications ○ Gas processing ○ Refinery ○ Petrochemical processes

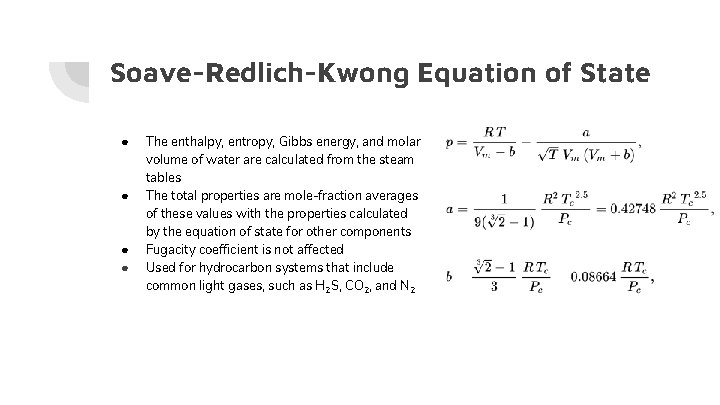
Soave-Redlich-Kwong Equation of State ● ● The enthalpy, entropy, Gibbs energy, and molar volume of water are calculated from the steam tables The total properties are mole-fraction averages of these values with the properties calculated by the equation of state for other components Fugacity coefficient is not affected Used for hydrocarbon systems that include common light gases, such as H 2 S, CO 2, and N 2

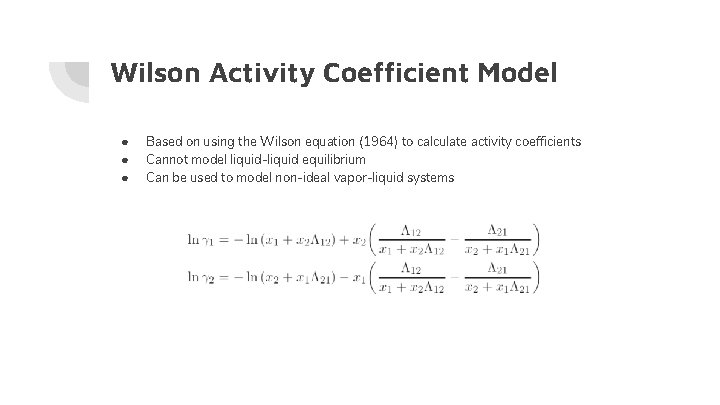
Wilson Activity Coefficient Model ● ● ● Based on using the Wilson equation (1964) to calculate activity coefficients Cannot model liquid-liquid equilibrium Can be used to model non-ideal vapor-liquid systems

UNIFAC and UNIQUAC Models ● ● ● The Universal Quasichemical (UNIQUAC) Model is an activity coefficient model that accounts for size and energy differences between molecules in a mixture. [1] The UNIQUAC Functional-group Activity Coefficient (UNIFAC) Model utilizes the contributions of functional groups within a molecule to estimate the size and energy differences. [1] Group Volume and Surface Area Parameters can be found in tables for use with the UNIFAC and UNIQUAC models

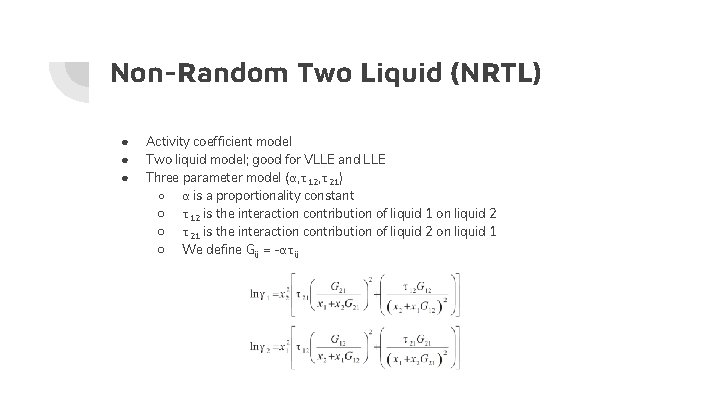
Non-Random Two Liquid (NRTL) ● ● ● Activity coefficient model Two liquid model; good for VLLE and LLE Three parameter model (α, τ12, τ21) ○ α is a proportionality constant ○ τ12 is the interaction contribution of liquid 1 on liquid 2 ○ τ21 is the interaction contribution of liquid 2 on liquid 1 ○ We define Gij = -ατij

Ideal Model ● ● Assumes all vapor phase equilibria exhibits ideal gas behavior and that all liquid phase equilibria exhibits properties of an ideal mixture The Equation of State used to model the vapor phase is the Ideal Gas Law ○ PV=n. RT An ideal mixture of liquids will have an activity coefficient of γ=1 The liquid phase equilibria can be explained by Raoult’s Law ○ Pi = Pivap xi

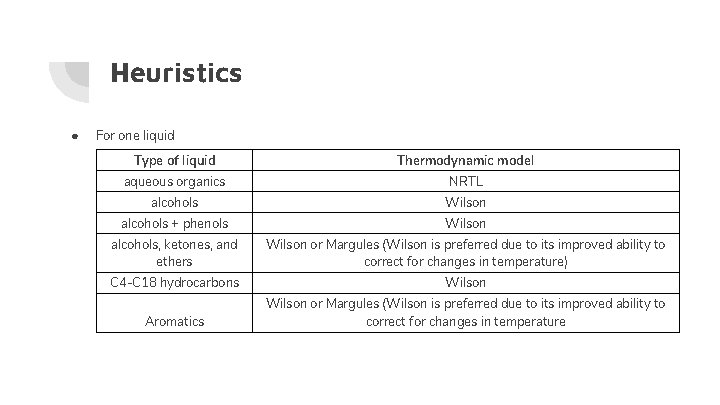
Heuristics ● For one liquid Type of liquid Thermodynamic model aqueous organics NRTL alcohols Wilson alcohols + phenols Wilson alcohols, ketones, and ethers Wilson or Margules (Wilson is preferred due to its improved ability to correct for changes in temperature) C 4 -C 18 hydrocarbons Wilson Aromatics Wilson or Margules (Wilson is preferred due to its improved ability to correct for changes in temperature

Cont. ● ● VLE ○ When in doubt for VLE calculations, use the Wilson equation. ○ Apply this rule under the assumption that binary interaction coefficients are available or can be estimated. LLE ○ Use the TK Wilson equation or the NRTL equation ○ Do not use Wilson ■ Not capable of performing LLE calculations

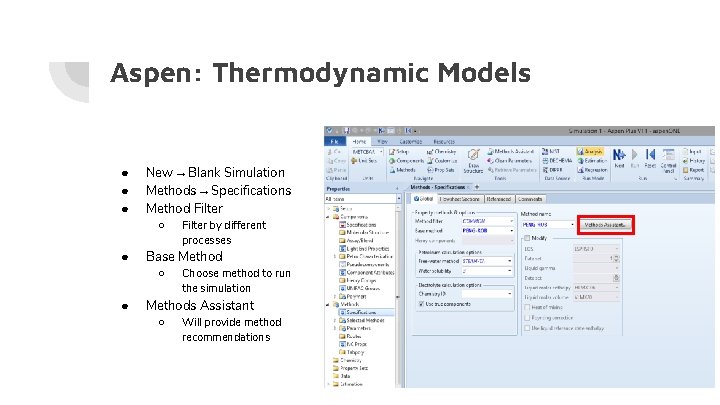
Aspen: Thermodynamic Models ● ● ● New → Blank Simulation Methods → Specifications Method Filter ○ ● Base Method ○ ● Filter by different processes Choose method to run the simulation Methods Assistant ○ Will provide method recommendations

Aspen: Methods Assistant

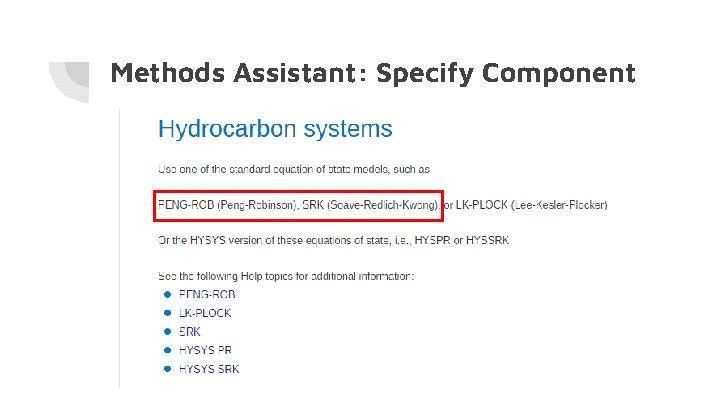
Methods Assistant: Specify Component

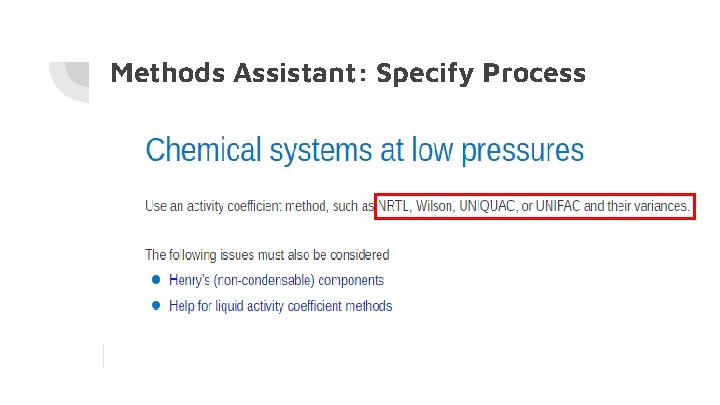
Methods Assistant: Specify Process

Aspen: Additional Information ● ● Using the Properties Environment ○ More detailed information about physical properties and EOS ○ Includes guideline charts on how to choose thermodynamic model Aspen Plus Reference ○ Detailed information about various EOS and activity coefficient models ○ Provides brief explanation on what models to use for different situations

Aspen: Aspen Plus Reference ● ● Aspen Plus Reference → Property Method Descriptions → Property Methods for Petroleum Mixtures → Petroleum Tuned Equation of States Property Methods ○ Peng-Robinson, SRK, etc. Aspen Plus Reference → Property Method Descriptions → Liquid Activity Coefficient Property Methods → Activity Coefficient Models ○ Wilson, NRTL, UNIFAC, etc

Best Models for Diesel ● ● Follow the Aspen Methods Assistant The Wilson model can handle any combination of polar and non-polar compounds, up to very strong nonideality (Aspen) ○ ● Proven by many research articles that Wilson is one of the best models to use in oil and gas simulation Thermodynamic model can be design optimization

References 1. Sandler, s. I. Chemical, Biomolecular, and Engineering Thermodynamics: Fifth Edition; John Wiley: Hoboken, NJ, 2017.