Asian Harmonization Working Party WORKING TOWARDS MEDICAL DEVICE












- Slides: 17

Asian Harmonization Working Party WORKING TOWARDS MEDICAL DEVICE HARMONIZATION IN ASIA Singapore Medical Device Regulations Marianne YAP Regulatory Scientist Medical Device Branch Therapeutics Products Division Health Products Regulation Group Health Sciences Authority 3 -6 th November 2008 New Delhi, India Copyright of the Health Sciences Authority 2008

Scope • Revisions / New Guidelines in the Last 1 -2 Years • Opportunity For Harmonization That Remains • Hurdles / Barriers To Harmonization • What really is Harmonization? 2 Copyright of the Health Sciences Authority 2008 2

Implementation of Medical Device Regulations Copyright of the Health Sciences Authority 2008

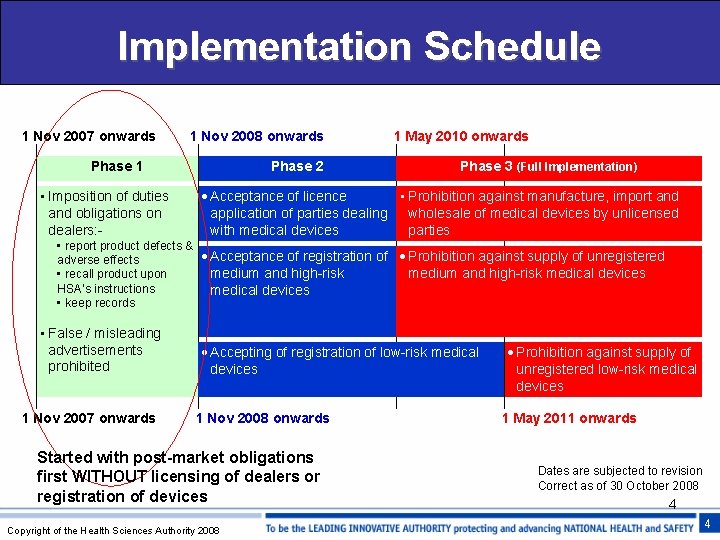
Implementation Schedule 1 Nov 2007 onwards 1 Nov 2008 onwards Phase 1 • Imposition of duties and obligations on dealers: - Phase 2 1 May 2010 onwards Phase 3 (Full Implementation) · Acceptance of licence • Prohibition against manufacture, import and application of parties dealing wholesale of medical devices by unlicensed with medical devices parties • report product defects & · Acceptance of registration of · Prohibition against supply of unregistered adverse effects medium and high-risk medical devices • recall product upon HSA’s instructions medical devices • keep records • False / misleading advertisements prohibited 1 Nov 2007 onwards · Accepting of registration of low-risk medical devices 1 Nov 2008 onwards Started with post-market obligations first WITHOUT licensing of dealers or registration of devices Copyright of the Health Sciences Authority 2008 · Prohibition against supply of unregistered low-risk medical devices 1 May 2011 onwards Dates are subjected to revision Correct as of 30 October 2008 4 4

Guidance Documents Finalized Guidance Documents 1) 2) 3) 4) 5) 6) 7) 8) 9) 10) 11) 12) 13) 14) Application of Good Distribution Practice for Medical Devices in Singapore Licensing for Manufacturers, Importers and Wholesalers of Medical Devices Preparation of a Site Master File for Licensing Medical Device Recall Reporting of Adverse Events for Medical Devices Distribution Records for Medical Devices Complaint Handling of Medical Devices Component Elements of a Dear Healthcare Professional Letter Medical Device Field Safety Corrective Action Risk Classification of General Medical Devices Risk Classification of In Vitro Diagnostic Medical Devices Medical Device Product Registration Letter of Authorisation Template Essential Principles for Safety and Performance of Medical Devices 5 Copyright of the Health Sciences Authority 2008 5

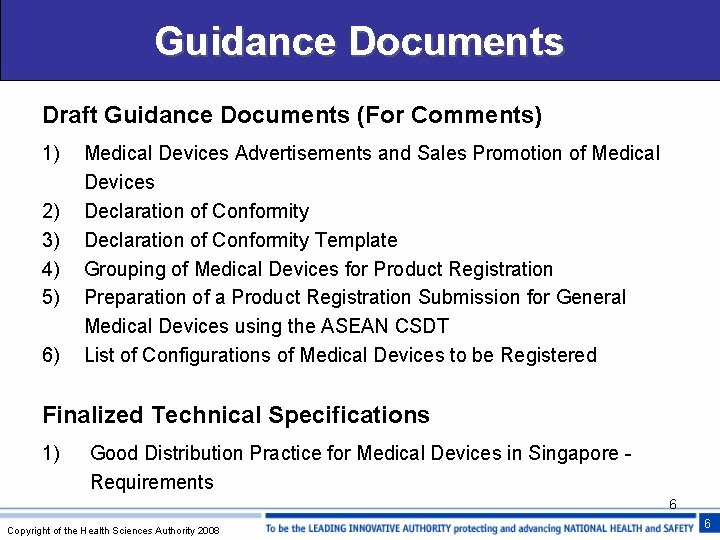
Guidance Documents Draft Guidance Documents (For Comments) 1) 2) 3) 4) 5) 6) Medical Devices Advertisements and Sales Promotion of Medical Devices Declaration of Conformity Template Grouping of Medical Devices for Product Registration Preparation of a Product Registration Submission for General Medical Devices using the ASEAN CSDT List of Configurations of Medical Devices to be Registered Finalized Technical Specifications 1) Good Distribution Practice for Medical Devices in Singapore Requirements 6 Copyright of the Health Sciences Authority 2008 6

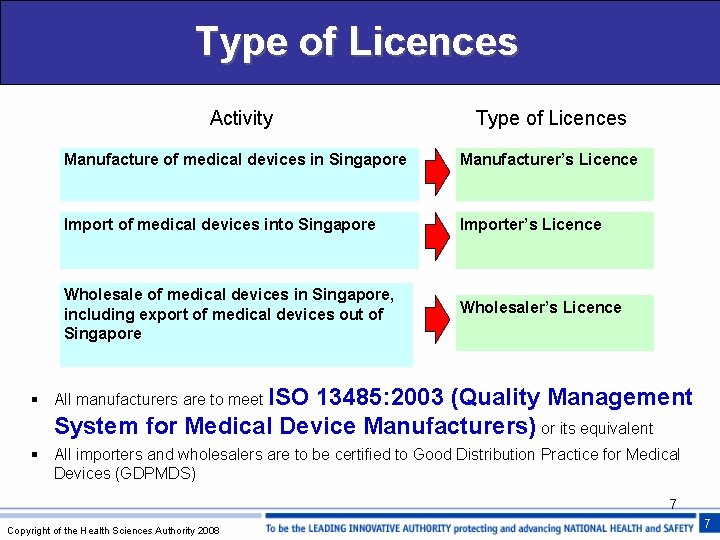
Type of Licences Activity Type of Licences Manufacture of medical devices in Singapore Manufacturer’s Licence Import of medical devices into Singapore Importer’s Licence Wholesale of medical devices in Singapore, including export of medical devices out of Singapore Wholesaler’s Licence § All manufacturers are to meet ISO 13485: 2003 (Quality Management System for Medical Device Manufacturers) or its equivalent § All importers and wholesalers are to be certified to Good Distribution Practice for Medical Devices (GDPMDS) 7 Copyright of the Health Sciences Authority 2008 7

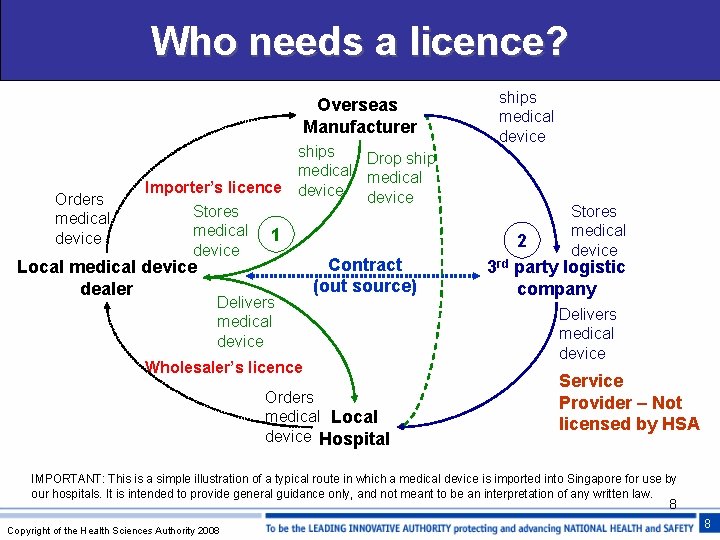
Who needs a licence? Overseas Manufacturer Orders medical device ships Drop ship medical Importer’s licence device Stores medical 1 device Local medical device dealer Delivers medical device Contract (out source) Wholesaler’s licence Orders medical Local device Hospital ships medical device Stores medical device 2 3 rd party logistic company Delivers medical device Service Provider – Not licensed by HSA IMPORTANT: This is a simple illustration of a typical route in which a medical device is imported into Singapore for use by our hospitals. It is intended to provide general guidance only, and not meant to be an interpretation of any written law. 8 Copyright of the Health Sciences Authority 2008 8

Opportunities for Harmonization Copyright of the Health Sciences Authority 2008

Opportunities for Harmonization Ø Risk Classification of General Medical Devices Ø Risk Classification of In Vitro Diagnostic Medical Devices Ø Essential Principles for Safety and Performance of Medical Devices Ø Definitions for Medical Device Recall, Field Safety Corrective Actions Ø Reporting Criteria of Adverse Events for Medical Devices Ø Grouping of Medical Devices for Product Registration Ø Preparation of Product Registration Submission to Regulatory Authorities Ø Elements for Conformity Assessment of Medical Devices Ø Adoption of ISO 13485 – QMS for Medical Devices Mfgers 10 Copyright of the Health Sciences Authority 2008 10

Hurdles to Harmonization Copyright of the Health Sciences Authority 2008

Hurdles to Harmonization 1. Differences in pace of harmonization among countries and regions – once legislation is in place, and local industry is accustomed to it, it will be time and resource intensive to effect changes. Linked and forms a cycle 2. Lack of harmonization in medical device manufacturing bases – countries that are importing and not manufacturing medical devices will always be deemed to be slow or reluctant to harmonize, especially when new changes are implemented in the manufacturing country! 12 Copyright of the Health Sciences Authority 2008 12

Understanding Harmonization Copyright of the Health Sciences Authority 2008

Understanding Harmonization Ø Harmonization: What is it really? Definition: to bring into consonance or accord (a. k. a. singing from the same song sheet) Ø Does harmonization mean: - (i) harmonized common technical requirements; OR (ii) one approval, many markets? (i. e. approval in one established jurisdiction, and others accept it without question? ) OR BOTH 14 Copyright of the Health Sciences Authority 2008 14

Understanding Harmonization Ø Ø “Technical” Harmonization: YES “Legal” Harmonization: NOT IMPOSSIBLE Requires: Legal Infrastructure; Political Will; Time for confidence building in each other’s system Only one real harmonized common market in this world – European Union, a unique economic and political partnership between 27 democratic European countries. EU Parliament (representing EU people), EU Council (representing national govts), EU Commission (representing common EU interests), EU Court of Justice, EU Directives, EU Committee for Standardization (CEN), EU harmonized standards, EU Notified Bodies) 15 Copyright of the Health Sciences Authority 2008 15

We Need Harmonization! Ø Technical Harmonization: YES, it can be done! Ø We Need Harmonization, and we need it URGENTLY! Ø Clarity in technical rules facilitates trade across borders, lowers costs of medical devices, promotes innovation, facilitates communications. 16 Copyright of the Health Sciences Authority 2008 16

www. hsa. gov. sg Copyright of the Health Sciences Authority 2008