ASDERA Overview Mission Develop novel drugs for unmet




- Slides: 15

ASDERA – Overview Mission Develop novel drugs for unmet needs by utilizing a computational biostatistics platform (US 7, 664, 616), the first to identify genetic risk factors for complex diseases Gauss-Legendre (~1800) Drug Discovery Platform Time-Pipeline © ASDERA (2016) 1900 Ohdner 9 Byte ANOVA Hoeffding (1948) 1965 IBM 256 k. B PCA 1985 PC 16 MB Bayes 2001 GPU 4 GB u-Stat Validation: Confirmation of known drug targets in epilepsy Proof-of-principle: L-fucose in Crohn’s disease (in phase 3) Currentlead: Mefenamic acid against mutism in autism (phase 3 ready) Current Next-in-line: Preventing metastases in breast cancer (pat. pending) Outlook: Delaying Alzheimer’s and Parkinson’s (pat. pending) Confidential Information 1

ASDERA – Lead Product Mission Lead Indication Product Status © ASDERA (2016) Develop novel drugs for unmet needs by utilizing a computational biostatistics platform (US 7, 664, 616), the first to identify genetic risk factors for complex diseases Disruption of Active Language Development (DALD) in toddlers developing Autism Spectrum Disorders (ASD) causing them to • become non-verbal and • develop life-long intellectual disability (ID) ASD-002: Market-exclusive ester-prodrug of Mefenamic Acid (MFA) Patent filed in US and EU. Orphan Drug Designation pending Preparing CMC / manufacturing for a short 505(b)(2) regulatory pathway for the single phase 2 b/3 trial needed for a breakthrough drug. Confidential Information 2

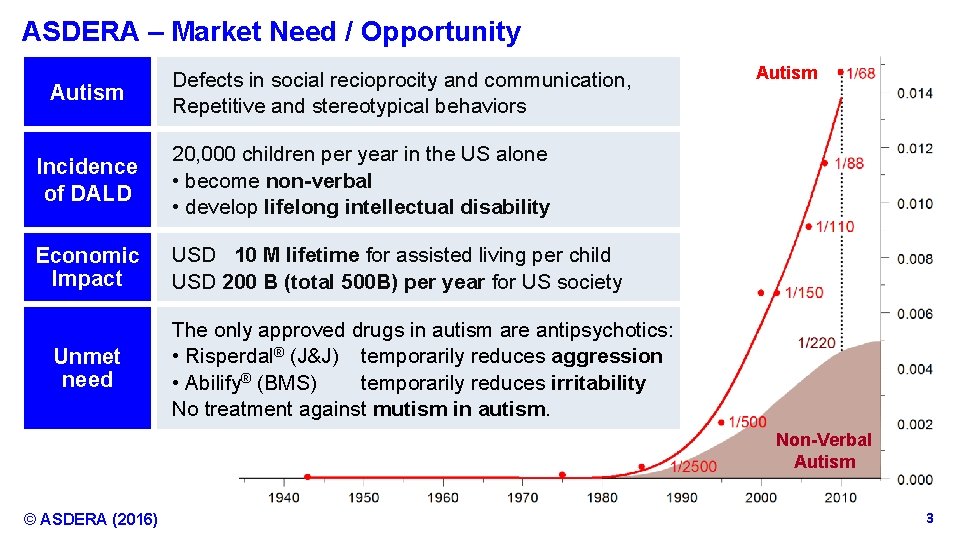
ASDERA – Market Need / Opportunity Autism Defects in social recioprocity and communication, Repetitive and stereotypical behaviors Incidence of DALD 20, 000 children per year in the US alone • become non-verbal • develop lifelong intellectual disability Economic Impact USD 10 M lifetime for assisted living per child USD 200 B (total 500 B) per year for US society Unmet need Autism The only approved drugs in autism are antipsychotics: • Risperdal® (J&J) temporarily reduces aggression • Abilify® (BMS) temporarily reduces irritability No treatment against mutism in autism. Non-Verbal Autism © ASDERA (2016) Confidential Information 3

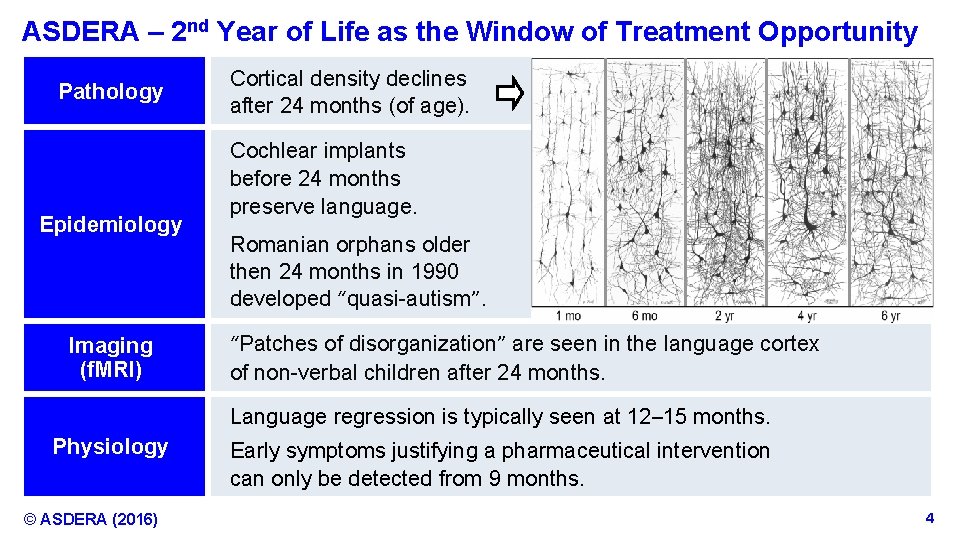
ASDERA – 2 nd Year of Life as the Window of Treatment Opportunity Pathology Epidemiology Imaging (f. MRI) Cortical density declines after 24 months (of age). Cochlear implants before 24 months preserve language. Romanian orphans older then 24 months in 1990 developed “quasi-autism”. “Patches of disorganization” are seen in the language cortex of non-verbal children after 24 months. Language regression is typically seen at 12– 15 months. Physiology © ASDERA (2016) Early symptoms justifying a pharmaceutical intervention can only be detected from 9 months. Confidential Information 4

ASDERA’s Novel Discovery Technology and Mefenamic Acid Hit Discovery Platform (US 7, 664, 616) The platform screens not only for individual genetic ‘letter’ positions (SNPs) but genetic ‘words’ (up to six neighboring SNPs). It also accounts for genetic ‘grammar’ (neighborhood, compound heterozygosity, recombination hotspots, . . . ) within the statistical method. Feasible only since 2001 (32 -bit OS), it already yielded several hits. npg Results from two independent ASD populations http: //www. nature. com/articles/tp 2013124 Genetic results Independent Confirmation Drug Hit © ASDERA (2016) showed lack-of-language associated with • Ion channels (excitation/inhibition imbalance), including • Known migraine genes (FHM). Guglielmi (2015) identified the same potassium (K+) channels and showed • gain-of-function in inward and • loss-of-function in outward K+ channels impairing ability of neurons to adjust to stress (hyperpolarization). Mefenamic acid (MFA) activates outward K+ channels. Confidential Information 5

ASDERA – in vitro / Animal / Clinical Evidence for Efficacy of MFA Pre-Clinical (Mode of Action) in vitro, MFA activates outward K+ channels. In mice, this reduction of hyperexcitability prevents induced seizures. Migraines and DALD share Migraineurs as a model for MFA • Individual and familial co-occurence • Genes (familial hemiplegic migraine, K+ channels) • Epileptiform EEGs • Avoidance of social contacts Clinical Trials of MFA Three clinical trials have shown that MFA is effective in migraine • treatment and • prevention US approval MFA is approved for the treatment of dysmenorrhea (incl. menstrual migraines with poor response to COX-NSAIDs). © ASDERA (2016) Confidential Information 6

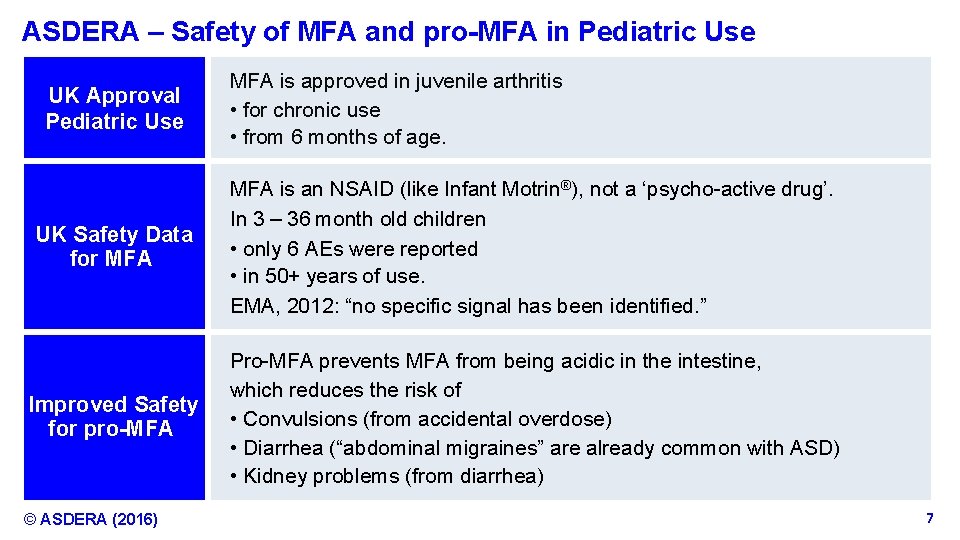
ASDERA – Safety of MFA and pro-MFA in Pediatric Use UK Approval Pediatric Use MFA is approved in juvenile arthritis • for chronic use • from 6 months of age. UK Safety Data for MFA is an NSAID (like Infant Motrin®), not a ‘psycho-active drug’. In 3 – 36 month old children • only 6 AEs were reported • in 50+ years of use. EMA, 2012: “no specific signal has been identified. ” Improved Safety for pro-MFA Pro-MFA prevents MFA from being acidic in the intestine, which reduces the risk of • Convulsions (from accidental overdose) ( • Diarrhea (“abdominal migraines” are already common with ASD) • Kidney problems (from diarrhea) © ASDERA (2016) Confidential Information 7

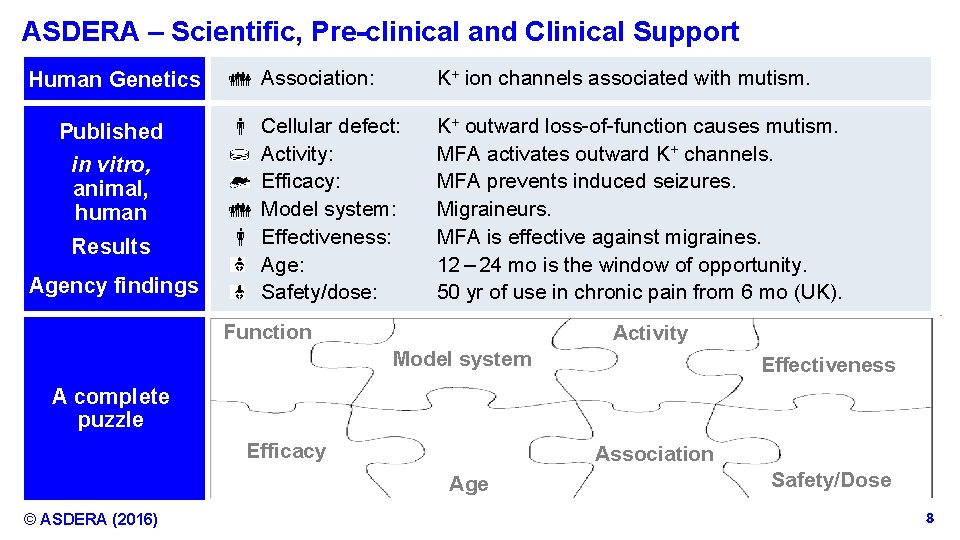
ASDERA – Scientific, Pre-clinical and Clinical Support Human Genetics Published in vitro, animal, human Results Agency findings Association: K+ ion channels associated with mutism. Cellular defect: Activity: Efficacy: Model system: Effectiveness: Age: Safety/dose: K+ outward loss-of-function causes mutism. MFA activates outward K+ channels. MFA prevents induced seizures. Migraineurs. MFA is effective against migraines. 12 – 24 mo is the window of opportunity. 50 yr of use in chronic pain from 6 mo (UK). Function Activity Model system Effectiveness A complete puzzle Efficacy Association Age © ASDERA (2016) Safety/Dose Confidential Information 8

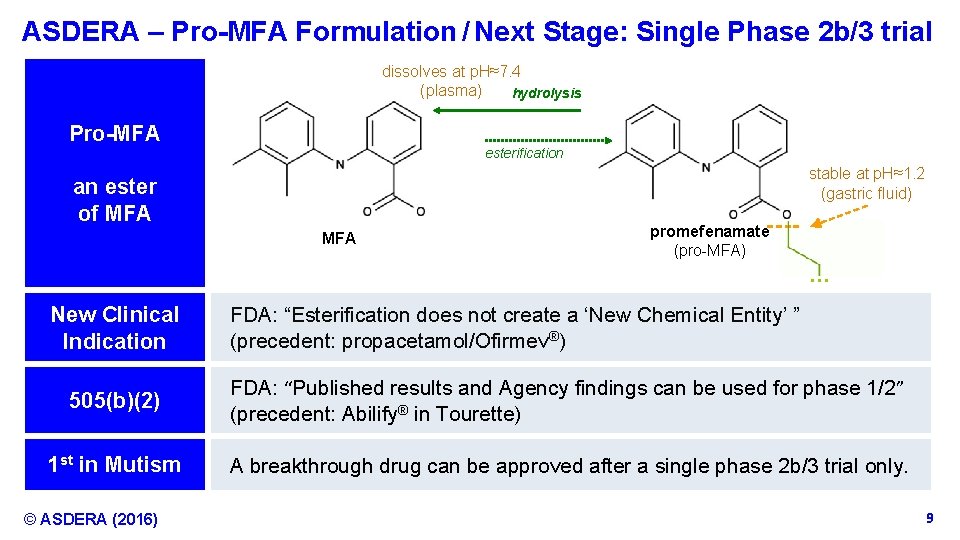
ASDERA – Pro-MFA Formulation / Next Stage: Single Phase 2 b/3 trial dissolves at p. H≈7. 4 (plasma) hydrolysis Pro-MFA esterification stable at p. H≈1. 2 (gastric fluid) an ester of MFA promefenamate (pro-MFA) … New Clinical Indication FDA: “Esterification does not create a ‘New Chemical Entity’ ” (precedent: propacetamol/Ofirmev®) 505(b)(2) FDA: “Published results and Agency findings can be used for phase 1/2” (precedent: Abilify® in Tourette) 1 st in Mutism A breakthrough drug can be approved after a single phase 2 b/3 trial only. © ASDERA (2016) Confidential Information 9

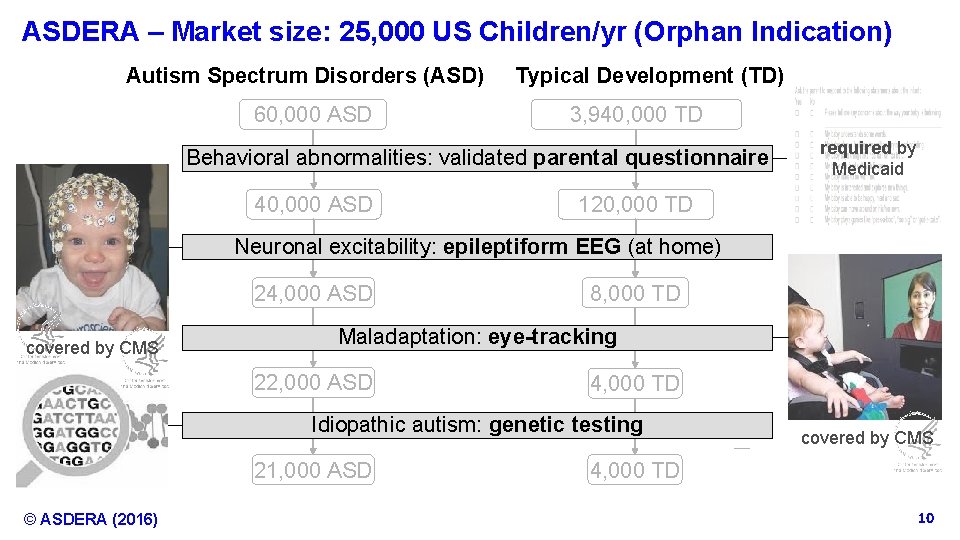
ASDERA – Market size: 25, 000 US Children/yr (Orphan Indication) Autism Spectrum Disorders (ASD) 60, 000 ASD Typical Development (TD) 3, 940, 000 TD Behavioralabnormalities: validatedparental questionnaire 40, 000 ASD required by Medicaid 120, 000 TD Neuronal EEG (at home) Neuronalexcitability: epileptiform. EEG(at (home) 24, 000 ASD covered by CMS 8, 000 TD Maladaptation: eye-tracking Maladaptation: 22, 000 ASD 4, 000 TD Idiopathic autism: genetic testing Idiopathic testing 21, 000 ASD © ASDERA (2016) covered by CMS 4, 000 TD Confidential Information 10

ASDERA – Clinical Phase 2 b/3 Trial Design / Funding Milestones Size / Duration Primary Endpoint 240 outpatient study (conducted by a contract research organization, CRO) 12 months to primary outcome (#words spoken NCT 01013545, see Ampyra®) At 3 months No words: Early exit to active drug (avoids child-specific IRB hurdles) Typical development: Treatment interruption (restart in case of regression). At 80 subjects Interim futility analysis (end of phase 2 b) Phase II $0. 25 M $0. 75 M © ASDERA (2016) $1. 0 M $2. 0 M Phase III $3. 5 M Confidential Information 11

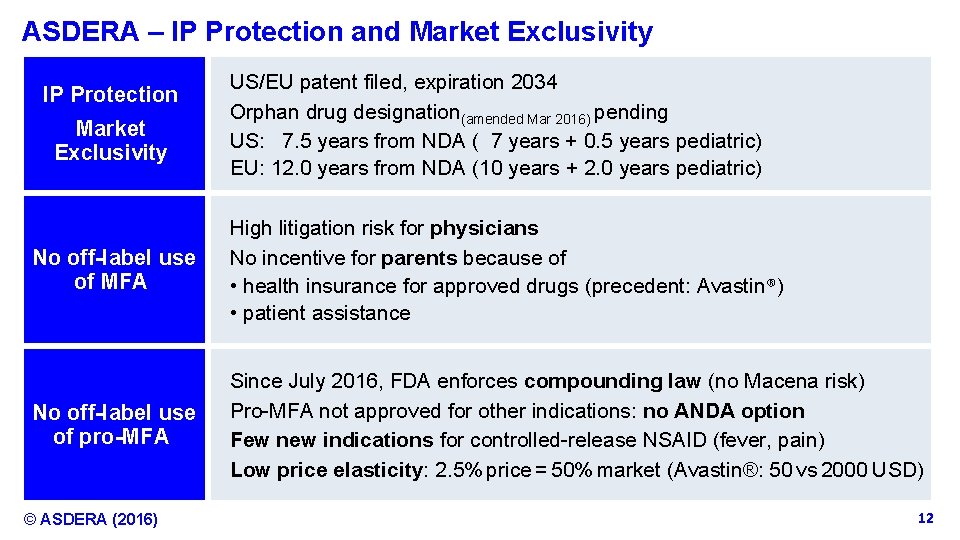
ASDERA – IP Protection and Market Exclusivity IP Protection Market Exclusivity US/EU patent filed, expiration 2034 Orphan drug designation(amended Mar 2016) pending US: 7. 5 years from NDA ( 7 years + 0. 5 years pediatric) EU: 12. 0 years from NDA (10 years + 2. 0 years pediatric) No off-label use of MFA High litigation risk for physicians No incentive for parents because of • health insurance for approved drugs (precedent: Avastin®) • patient assistance No off-label use of pro-MFA Since July 2016, FDA enforces compounding law (no Macena risk) Pro-MFA not approved for other indications: no ANDA option Few new indications for controlled-release NSAID (fever, pain) Low price elasticity: 2. 5% price = 50% market (Avastin®: 50 vs 2000 USD) © ASDERA (2016) Confidential Information 12

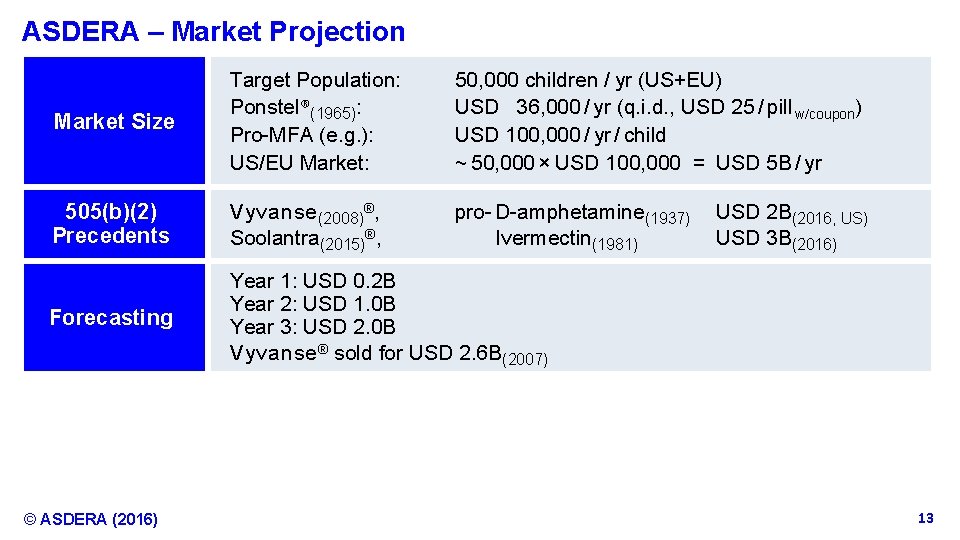
ASDERA – Market Projection Market Size Target Population: Ponstel®(1965): Pro-MFA (e. g. ): US/EU Market: 50, 000 children / yr (US+EU) USD 36, 000 / yr (q. i. d. , USD 25 / pill w/coupon) USD 100, 000 / yr / child ~ 50, 000 × USD 100, 000 = USD 5 B / yr 505(b)(2) Precedents V y v a n s e (2008)®, Soolantra(2015)®, pro- D-amphetamine(1937) Ivermectin(1981) Forecasting Year 1: USD 0. 2 B Year 2: USD 1. 0 B Year 3: USD 2. 0 B V y v a n s e ® sold for USD 2. 6 B(2007) © ASDERA (2016) USD 2 B(2016, US) USD 3 B(2016) Confidential Information 13

ASDERA – Virtual Company Team / Partners* Management: in. Ventiv Health Clinical will lead FDA interactions from pre. IND to NDA and assume all management responsibilities during the trial. Therapeutic areas: generics / pediatrics Phase III: full range of clinical trial services Consulting: small molecules / 505(b)(2) Knut M. Wittkowski, Ph. D, Sc. D Senior Research Associate, The Rockefeller Univ. Director, Biostatistics, Epidemiology & Research Design Center for Clinical and Translational Science apl. Professor, Eberhard-Karls-University, Tübingen http: //www. inventivhealthclinical. com/ Drug Development: Regis Technologies Gabrielle Gold-von Simson, MD, MSc Partners with pharmaceutical/biotech companies to help expedite drugs to market c. GMP Custom Services Assistant Professor of Pediatrics Medical Director, Inpatient Pediatrics Director Clinical Research Center, NYU School of Medicine PI, Drug Development Educational Programm (NIDDK) New York Univ. Langone Medical Center http: //www. registech. com/ Regulatory Affairs: Bert Spilker, Ph. D, MD John Jay Gargus, MD, Ph. D Independent Consultant on Regulatory Affairs Clin. Professor of Pharmacy Practice, U of Minnesota Sr. VP Scientific/Regulatory Affairs, Ph. RMA (1998– 2001) President/Co-founder Orphan Medical, Inc (1993– 2005) Professor of Pediatrics, Physiology & Biophysics Director, Center for Autism Research and Translation PI, Drug Discovery Platform for Autism Univ. California Irvine © ASDERA (2016) http: //www. bertspilker. com/ * Terms being finalized Confidential Information 14

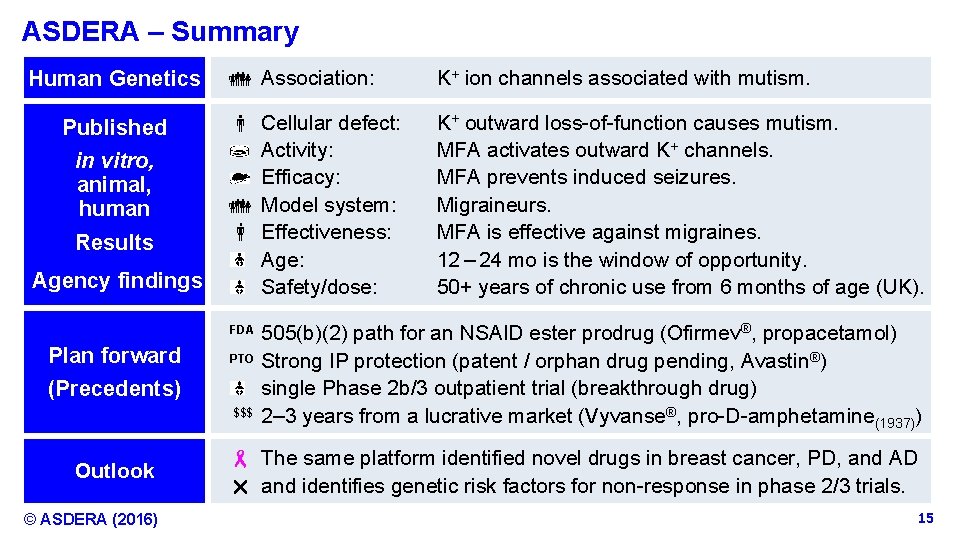
ASDERA – Summary Human Genetics Published in vitro, animal, human Results Agency findings Association: K+ ion channels associated with mutism. Cellular defect: Activity: Efficacy: Model system: Effectiveness: Age: Safety/dose: K+ outward loss-of-function causes mutism. MFA activates outward K+ channels. MFA prevents induced seizures. Migraineurs. MFA is effective against migraines. 12 – 24 mo is the window of opportunity. 50+ years of chronic use from 6 months of age (UK). FDA Plan forward PTO (Precedents) $$$ Outlook © ASDERA (2016) 505(b)(2) path for an NSAID ester prodrug (Ofirmev®, propacetamol) Strong IP protection (patent / orphan drug pending, Avastin®) single Phase 2 b/3 outpatient trial (breakthrough drug) 2– 3 years from a lucrative market (Vyvanse®, pro-D-amphetamine(1937)) - The same platform identified novel drugs in breast cancer, PD, and AD r and identifies genetic risk factors for non-response in phase 2/3 trials. Confidential Information 15
 Asdera
Asdera Unmet needs in severe asthma
Unmet needs in severe asthma Unmet financial need
Unmet financial need Fredsgudinnan
Fredsgudinnan Orubbliga rättigheter
Orubbliga rättigheter Bamse för de yngsta
Bamse för de yngsta Densitet vatten
Densitet vatten Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Tillitsbaserad ledning
Tillitsbaserad ledning Bat mitza
Bat mitza Nationell inriktning för artificiell intelligens
Nationell inriktning för artificiell intelligens Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Sju för caesar
Sju för caesar Mästar lärling modellen
Mästar lärling modellen Matematisk modellering eksempel
Matematisk modellering eksempel Adressändring ideell förening
Adressändring ideell förening




























