As always n n OWL LonCapa assignments n



- Slides: 7

As always… n n OWL Lon-Capa assignments n n HW: equilibrium (due Monday) Quiz: Mon-Wed next week Lecture videos Textbook n n Read Do text homework 1

Exam I n n Thursday, September 26, 7: 00 -9: 00 pm; rooms are on the website. Conflict: (9/26) 4: 30 -6: 30 pm in 140 Burrill; sign up in 1026 CA (starting 9/19) Conflict with conflict? Email me right away. Review sessions: n n Sunday (9/22): 165 Noyes Lab (4 -6 pm) Monday (9/23): 1024 Chemistry Annex (6 -8 pm) 2

Exam I n Practice exam will be posted after lecture. n n n Take when you feel ready for the exam. Take as an actual exam (not just more practice problems). See the front of the exam for information that you are given and the number of problems. 3

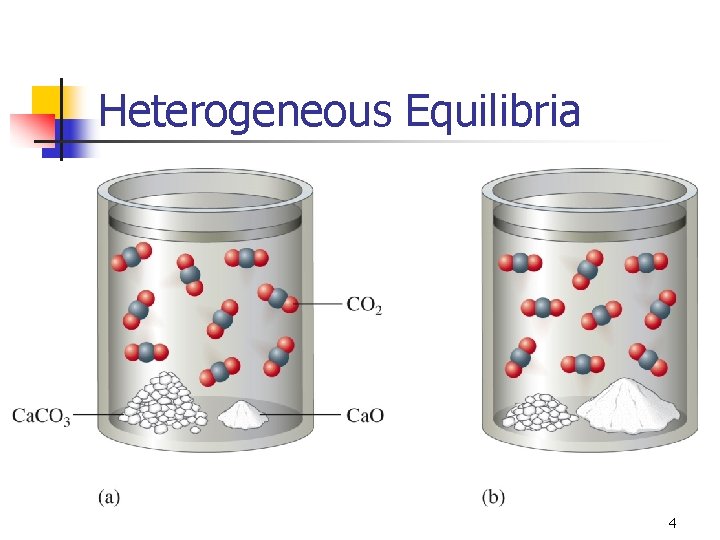
Heterogeneous Equilibria 4

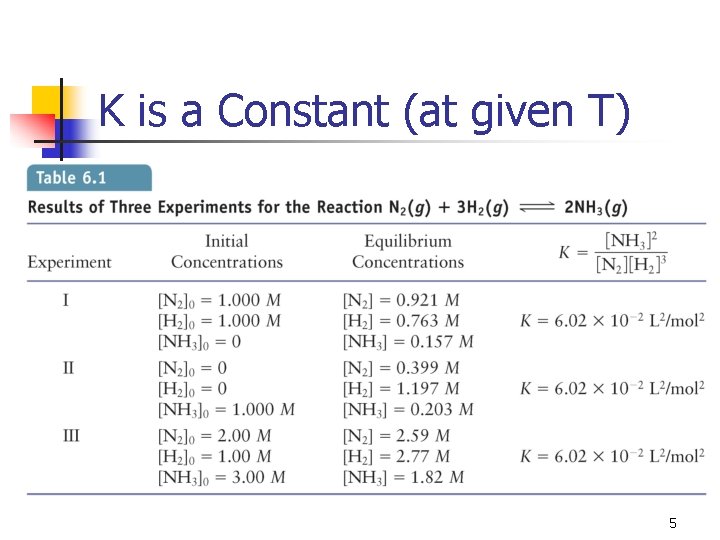
K is a Constant (at given T) 5

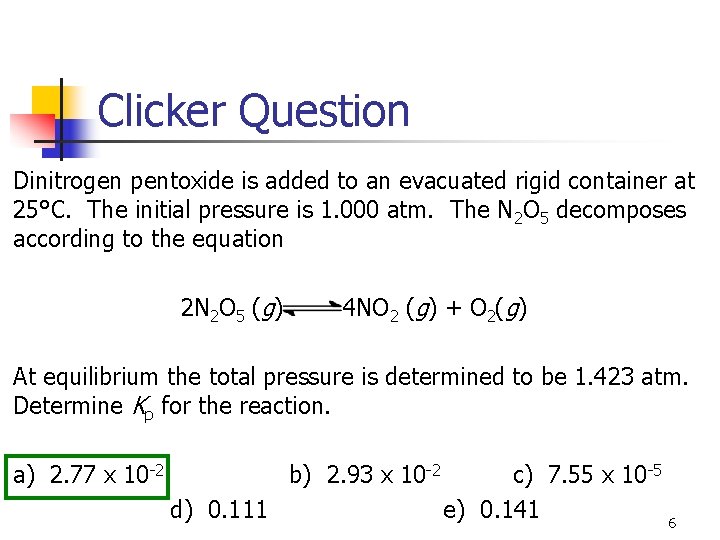
Clicker Question Dinitrogen pentoxide is added to an evacuated rigid container at 25°C. The initial pressure is 1. 000 atm. The N 2 O 5 decomposes according to the equation 2 N 2 O 5 (g) 4 NO 2 (g) + O 2(g) At equilibrium the total pressure is determined to be 1. 423 atm. Determine Kp for the reaction. a) 2. 77 x 10 -2 b) 2. 93 x 10 -2 d) 0. 111 c) 7. 55 x 10 -5 e) 0. 141 6

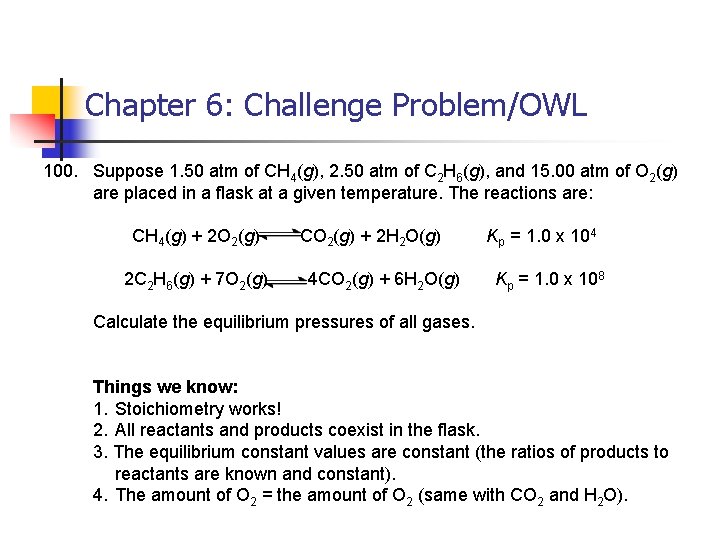
Chapter 6: Challenge Problem/OWL 100. Suppose 1. 50 atm of CH 4(g), 2. 50 atm of C 2 H 6(g), and 15. 00 atm of O 2(g) are placed in a flask at a given temperature. The reactions are: CH 4(g) + 2 O 2(g) 2 C 2 H 6(g) + 7 O 2(g) CO 2(g) + 2 H 2 O(g) 4 CO 2(g) + 6 H 2 O(g) Kp = 1. 0 x 104 Kp = 1. 0 x 108 Calculate the equilibrium pressures of all gases. Things we know: 1. Stoichiometry works! 2. All reactants and products coexist in the flask. 3. The equilibrium constant values are constant (the ratios of products to reactants are known and constant). 4. The amount of O 2 = the amount of O 2 (same with CO 2 and H 2 O).