As always n n OWL LonCapa assignments Lecture




- Slides: 8

As always… n n OWL Lon-Capa assignments Lecture videos Textbook n n Read Do text homework 1

OWL Question 3 (#34 Chapter 10) Calculate the change in entropy that occurs when 18. 02 g of ice at – 10. 0°C is placed in 54. 05 g of water at 100. 0°C in a perfectly insulated vessel. Assume that the molar heat capacities for and are 37. 5 J/Kmol and 75. 3 J/Kmol , respectively, and the molar enthalpy of fusion for ice is 6. 01 k. J/mol. 2

Clicker Question Given the following enthalpies of formation, determine the enthalpy of vaporization of methanol (CH 3 OH): CH 3 OH(l) CH 3 OH(g) ΔHf° (k. J/mol) – 239 – 201 a) 38 k. J/mol b) – 38 k. J/mol c) 440 k. J/mol d) – 440 k. J/mol e) I do not know. 3

Clicker Question Given the following absolute entropies, determine the value of ΔS° for methanol (CH 3 OH): CH 3 OH(l) CH 3 OH(g) S° (J/Kmol) 127 240 a) – 113 J/Kmol b) 113 J/Kmol c) – 367 J/Kmol d) 367 J/Kmol e) I do not know. 4

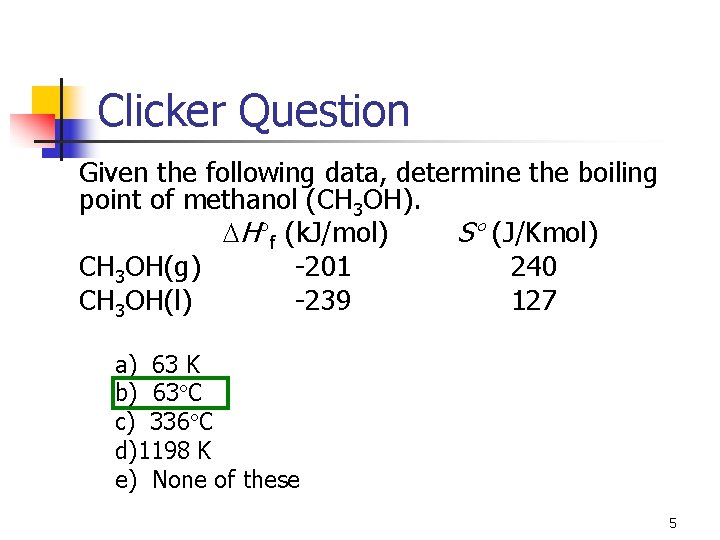
Clicker Question Given the following data, determine the boiling point of methanol (CH 3 OH). H f (k. J/mol) S (J/Kmol) CH 3 OH(g) -201 240 CH 3 OH(l) -239 127 a) 63 K b) 63 C c) 336 C d)1198 K e) None of these 5

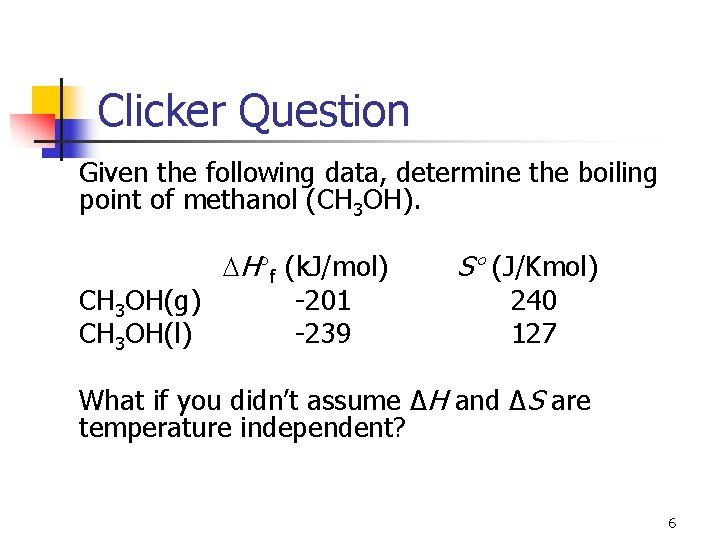
Clicker Question Given the following data, determine the boiling point of methanol (CH 3 OH). H f (k. J/mol) CH 3 OH(g) -201 CH 3 OH(l) -239 S (J/Kmol) 240 127 What if you didn’t assume ΔH and ΔS are temperature independent? 6

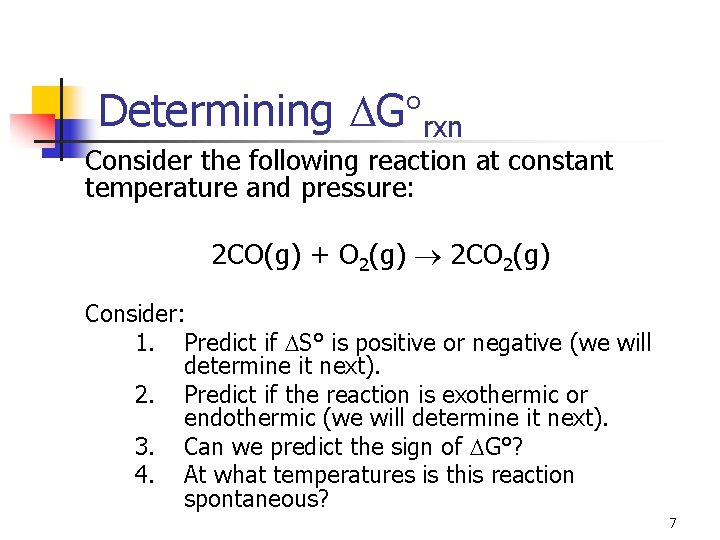
Determining G rxn Consider the following reaction at constant temperature and pressure: 2 CO(g) + O 2(g) 2 CO 2(g) Consider: 1. Predict if S° is positive or negative (we will determine it next). 2. Predict if the reaction is exothermic or endothermic (we will determine it next). 3. Can we predict the sign of G°? 4. At what temperatures is this reaction spontaneous? 7

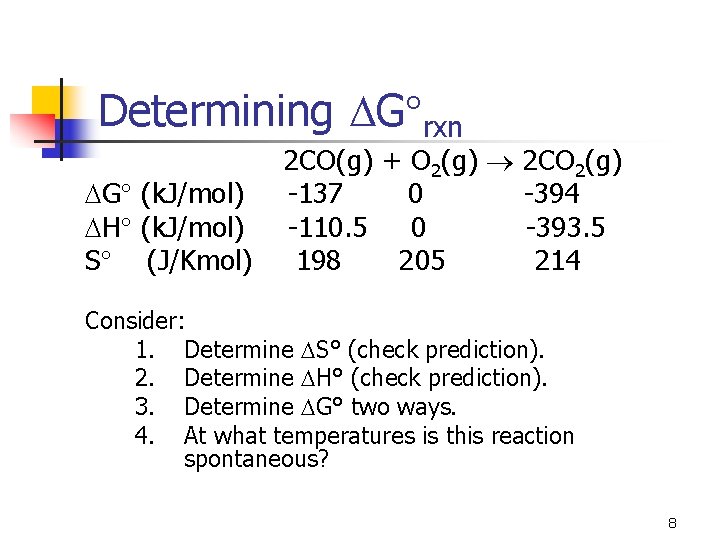
Determining G rxn 2 CO(g) + O 2(g) 2 CO 2(g) G (k. J/mol) -137 0 -394 H (k. J/mol) -110. 5 0 -393. 5 S (J/Kmol) 198 205 214 Consider: 1. Determine S° (check prediction). 2. Determine H° (check prediction). 3. Determine G° two ways. 4. At what temperatures is this reaction spontaneous? 8