As always n n n LonCapa assignments Lecture




- Slides: 10

As always… n n n Lon-Capa assignments Lecture videos Textbook n n Read Do text homework 1

Three Big Topics n n n Stoichiometry: information on “what” happens; allows us to determine amounts of reactants and products. Thermodynamics: information on “why” reactions happen; allows us to make predictions about whether a reaction will occur. Kinetics: information on “how” reactions happen (mechanisms); relates to the 2 speed of reactions.

Clicker Question A diamond will eventually turn to graphite, although the process takes so long we do not observe it. Which of the following is true about diamond and graphite? a) Diamond and graphite are both considered thermodynamically stable relative to one another. b) Diamond is kinetically unstable, but it is thermodynamically stable relative to graphite. c) Diamond is kinetically stable and graphite is kinetically unstable. d) Diamond is both kinetically unstable and thermodynamically unstable relative to graphite. e) Diamond is kinetically stable, but it is thermodynamically unstable relative to graphite. 3

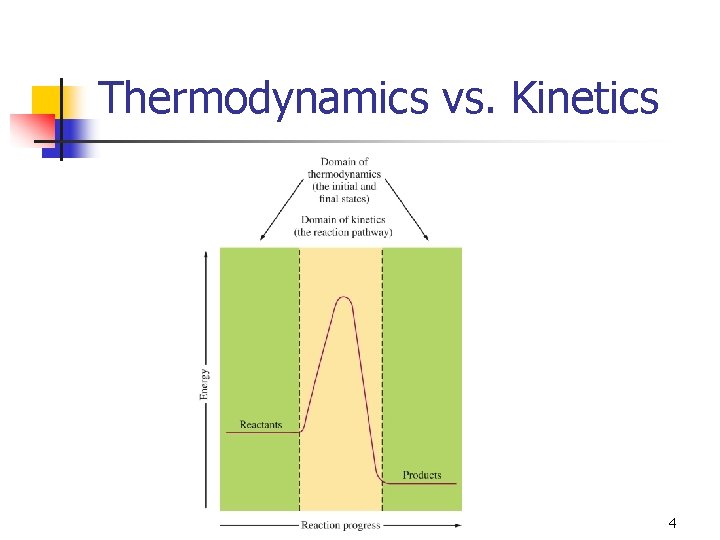
Thermodynamics vs. Kinetics 4

Mechanisms n Series of elementary steps. n n Must be chemically reasonable n n Must have the correct stoichiometry. That is, the steps must add up to the overall reaction. How can we tell this? Cannot prove but can support empirically. n Must agree with the overall rate law. 5

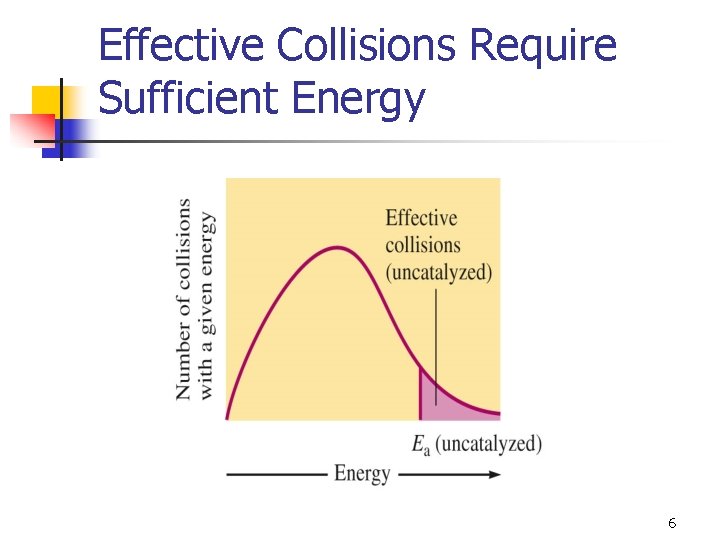
Effective Collisions Require Sufficient Energy 6

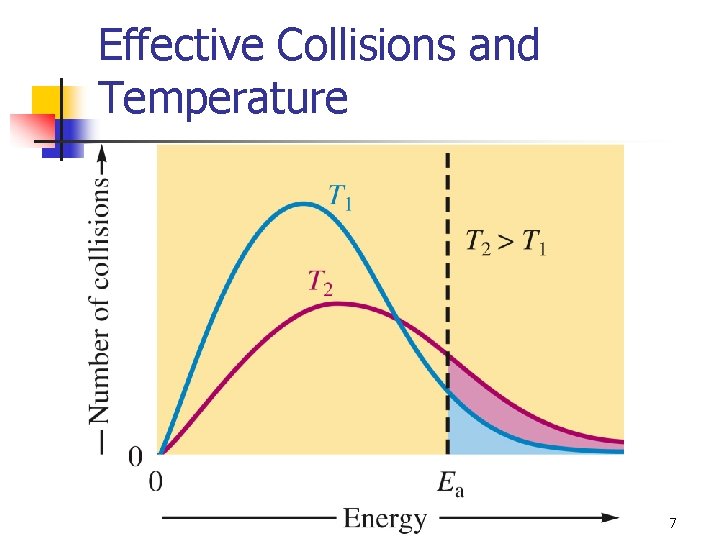
Effective Collisions and Temperature 7

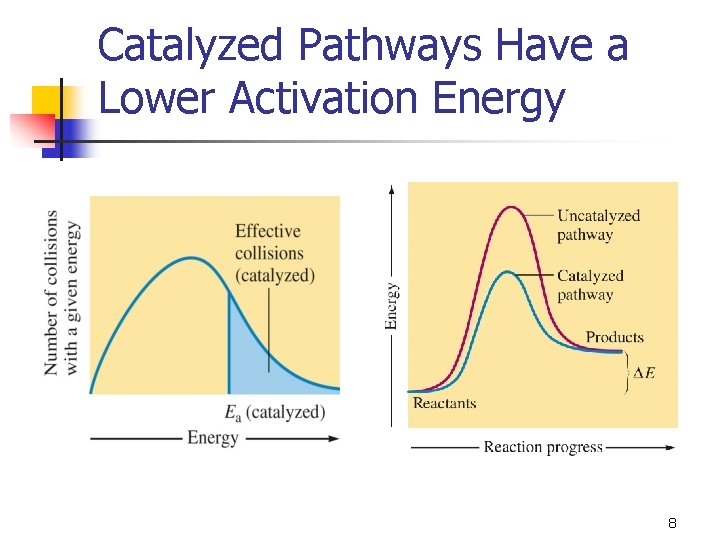
Catalyzed Pathways Have a Lower Activation Energy 8

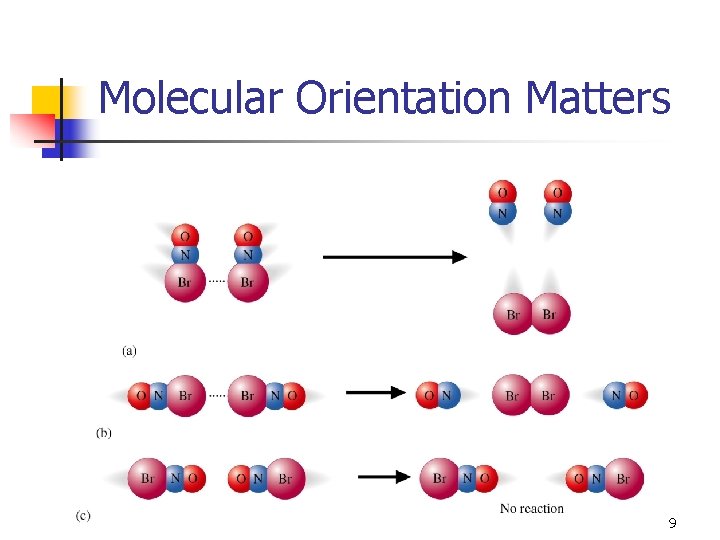
Molecular Orientation Matters 9

Questions to Consider… n n n What do we mean by rate? How can we measure rate? How do we determine the order of a reaction? How do we calculate the rate constant, k? How do we calculate the activation energy, Ea, of a reaction? 10