As always n n n LonCapa assignments Lecture






- Slides: 7

As always… n n n Lon-Capa assignments Lecture videos Textbook n n Read Do text homework 1

Exam II n n Thursday, November 2, 7: 00 -9: 00 pm; rooms are on the website. Conflict: (11/2) 4: 30 -6: 30 pm in 101 Transportation Building; sign up in 1026 CA Conflict with conflict? Email me right away. Review sessions: n n Monday (10/30): 161 Noyes Lab; 7 -9 pm Tuesday (10/31): 1024 Chem Annex; 7 -9 pm 2

Exam II n Practice exam now posted. . n n n Take when you feel ready for the exam. Take as an actual exam (not just more practice problems). See the front of the exam for information that you are given and the number of problems. 3

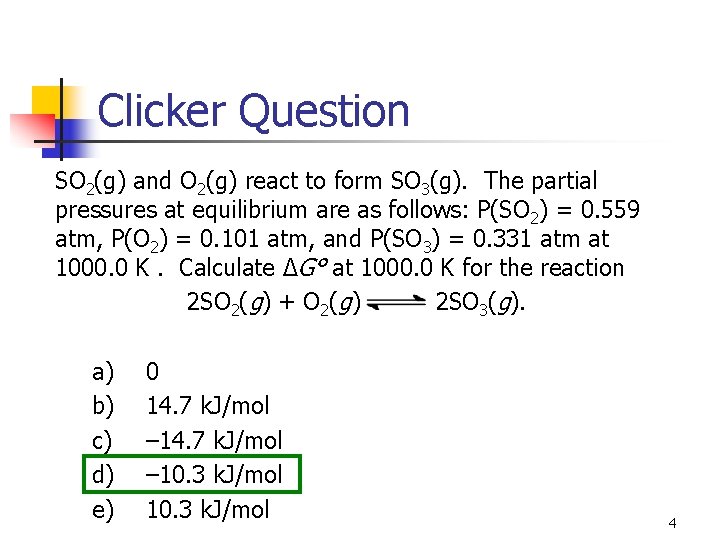
Clicker Question SO 2(g) and O 2(g) react to form SO 3(g). The partial pressures at equilibrium are as follows: P(SO 2) = 0. 559 atm, P(O 2) = 0. 101 atm, and P(SO 3) = 0. 331 atm at 1000. 0 K. Calculate ΔG° at 1000. 0 K for the reaction 2 SO 2(g) + O 2(g) 2 SO 3(g). a) b) c) d) e) 0 14. 7 k. J/mol – 10. 3 k. J/mol 4

Clicker Question For the reaction 2 HF(g) H 2(g) + F 2(g), G° = 38. 3 k. J, at 1000 K. If, at this temperature, 5. 00 mol of HF(g), 0. 500 mol of H 2(g), and 0. 75 mol of F 2(g) are mixed in a 1. 00 -L container: a) b) c) d) e) Some HF will decompose (to yield H 2 and F 2). Some HF will be formed (from H 2 and F 2). The system is at equilibrium. Not enough data are given to answer this question. I do not know. 5

Clicker Question (1/2) Given that for H 2 O(l), Gf = ‒ 237. 0 k. J/mol, determine the value of the equilibrium constant at 25. 0 C for the reaction: 2 H 2(g) + O 2(g) 2 H 2 O(l). a) b) c) d) e) 1. 10 6. 83 x 1024 3. 50 x 1041 1. 22 x 1083 I do not know. 6

Clicker Question (2/2) How would the value of K change if the reaction was carried out at 90. 0 C? The value of K 90 would be _______ than the value of K 25 because the reaction is ______. 2 H 2(g) + O 2(g) 2 H 2 O(l). a) b) c) d) e) greater, exothermic greater, endothermic less, exothermic less, endothermic The value of K would not change (it is, after all, called the equilibrium constant). 7