As always n n n LonCapa assignments Lecture


- Slides: 7

As always… n n n Lon-Capa assignments Lecture videos Textbook n n Read Do text homework 1

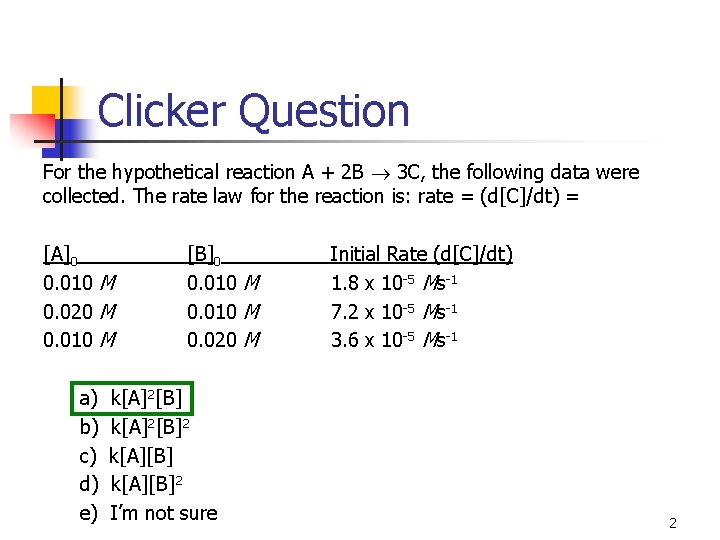
Clicker Question For the hypothetical reaction A + 2 B 3 C, the following data were collected. The rate law for the reaction is: rate = (d[C]/dt) = [A]0 0. 010 M 0. 020 M 0. 010 M a) b) c) d) e) [B]0 0. 010 M 0. 020 M k[A]2[B]2 k[A][B]2 I’m not sure Initial Rate (d[C]/dt) 1. 8 x 10 -5 Ms-1 7. 2 x 10 -5 Ms-1 3. 6 x 10 -5 Ms-1 2

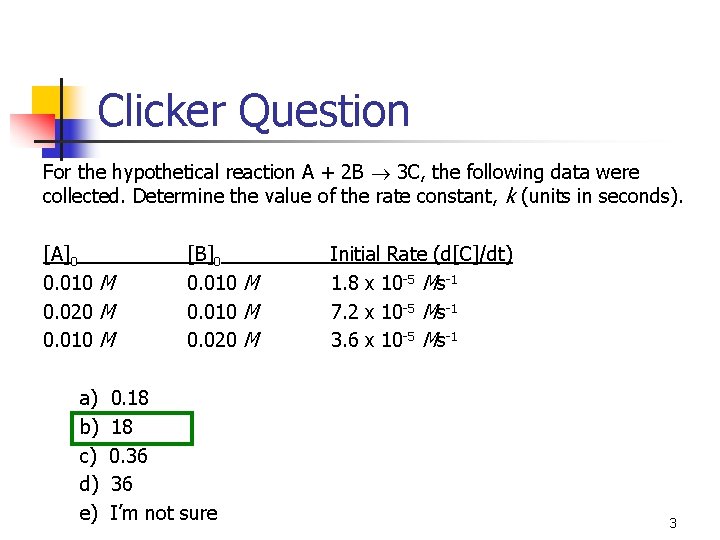
Clicker Question For the hypothetical reaction A + 2 B 3 C, the following data were collected. Determine the value of the rate constant, k (units in seconds). [A]0 0. 010 M 0. 020 M 0. 010 M a) b) c) d) e) [B]0 0. 010 M 0. 020 M 0. 18 18 0. 36 36 I’m not sure Initial Rate (d[C]/dt) 1. 8 x 10 -5 Ms-1 7. 2 x 10 -5 Ms-1 3. 6 x 10 -5 Ms-1 3

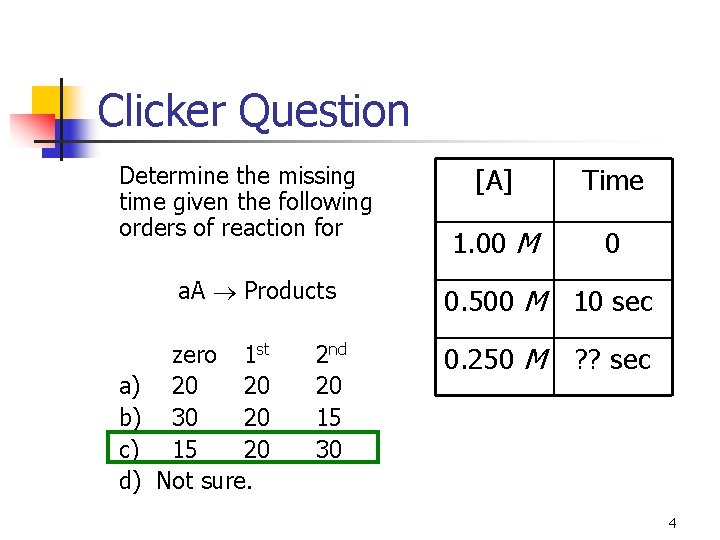
Clicker Question Determine the missing time given the following orders of reaction for a. A Products a) b) c) d) zero 1 st 20 20 30 20 15 20 Not sure. 2 nd 20 15 30 [A] Time 1. 00 M 0 0. 500 M 10 sec 0. 250 M ? ? sec 4

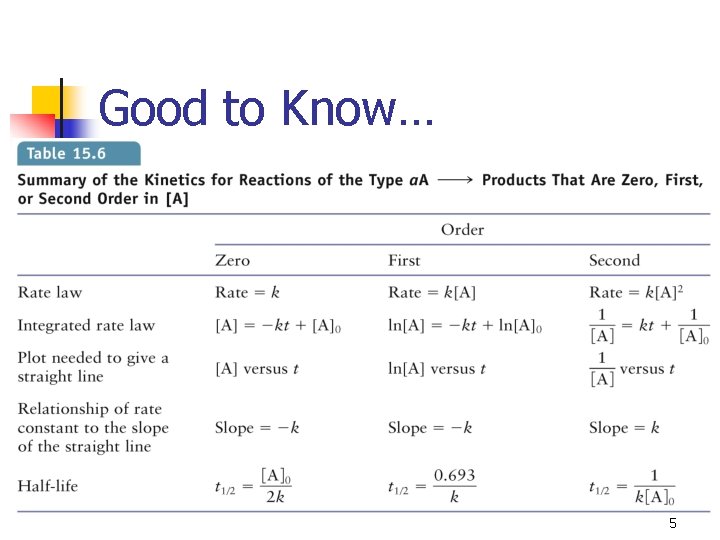
Good to Know… 5

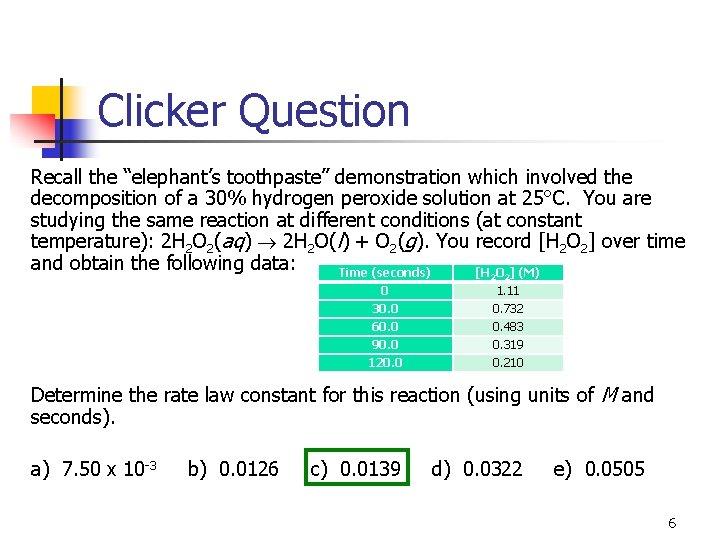
Clicker Question Recall the “elephant’s toothpaste” demonstration which involved the decomposition of a 30% hydrogen peroxide solution at 25°C. You are studying the same reaction at different conditions (at constant temperature): 2 H 2 O 2(aq) 2 H 2 O(l) + O 2(g). You record [H 2 O 2] over time and obtain the following data: Time (seconds) [H O ] (M) 2 0 30. 0 60. 0 90. 0 120. 0 2 1. 11 0. 732 0. 483 0. 319 0. 210 Determine the rate law constant for this reaction (using units of M and seconds). a) 7. 50 x 10 -3 b) 0. 0126 c) 0. 0139 d) 0. 0322 e) 0. 0505 6

Clicker Question A first-order reaction is 42% complete at the end of 17 minutes. Determine the value of the rate constant. a) 3. 2 x 10 -2 min-1 b) 5. 1 x 10 -2 min-1 c) 1. 8 min-1 d) 20. min-1 e) 31 min-1 7











