Arterial Blood Gas Interpretation AcidBase Balance n n

Arterial Blood Gas Interpretation
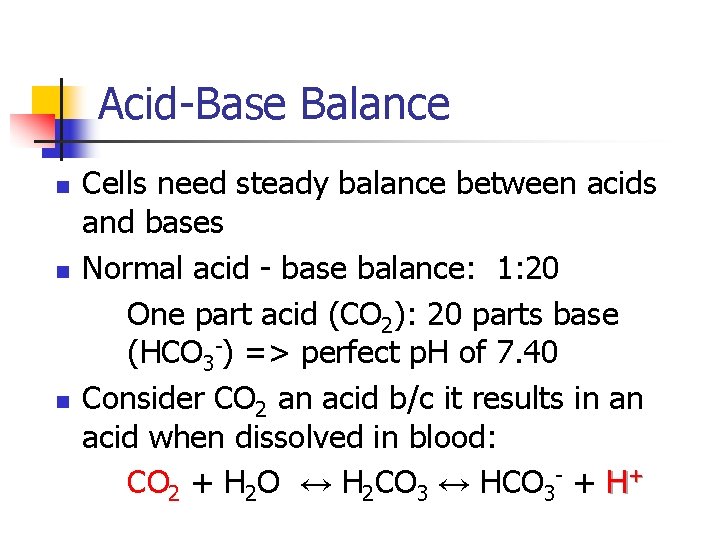
Acid-Base Balance n n n Cells need steady balance between acids and bases Normal acid - base balance: 1: 20 One part acid (CO 2): 20 parts base (HCO 3 -) => perfect p. H of 7. 40 Consider CO 2 an acid b/c it results in an acid when dissolved in blood: CO 2 + H 2 O ↔ H 2 CO 3 ↔ HCO 3 - + H+

Acid-Base Balance n Acid gain or base loss => Acidosis n n (p. H < 7. 40) Gain base or lose acids => Alkalosis n (ph > 7. 40)
Regulation of Acid-Base Balance n Regulatory mechanisms are very sensitive to small changes in p. H n n n Buffers Respiratory System Renal System
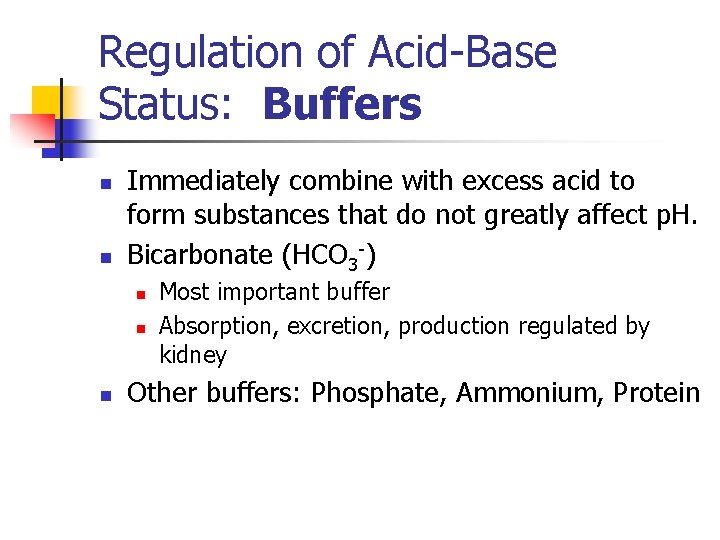
Regulation of Acid-Base Status: Buffers n n Immediately combine with excess acid to form substances that do not greatly affect p. H. Bicarbonate (HCO 3 -) n n n Most important buffer Absorption, excretion, production regulated by kidney Other buffers: Phosphate, Ammonium, Protein
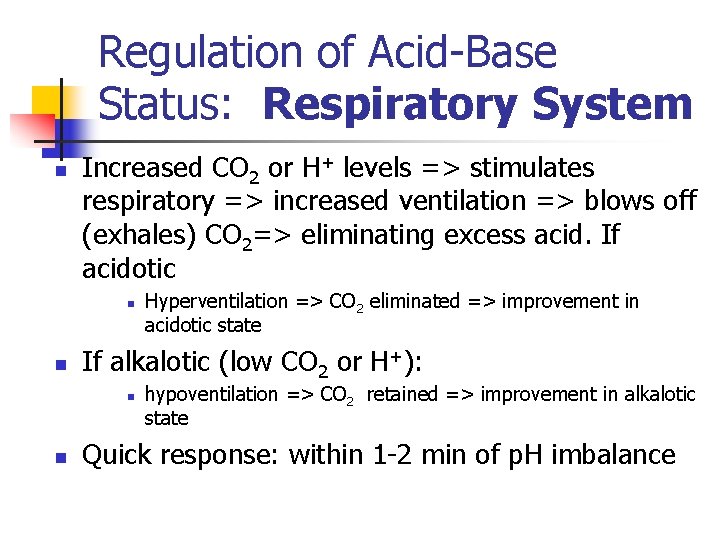
Regulation of Acid-Base Status: Respiratory System n Increased CO 2 or H+ levels => stimulates respiratory => increased ventilation => blows off (exhales) CO 2=> eliminating excess acid. If acidotic n n If alkalotic (low CO 2 or H+): n n Hyperventilation => CO 2 eliminated => improvement in acidotic state hypoventilation => CO 2 retained => improvement in alkalotic state Quick response: within 1 -2 min of p. H imbalance
Regulation of Acid-Base Status: Renal System n Kidneys conserve and/or eliminate H+ and HCO 3 - in response to abnormal p. H n n n If acidotic => eliminate H+ (acid) and retain HCO 3 - (base) in effort to normalize p. H If alkalotic => Eliminate HCO 3 - (base) in effort to normalize p. H Response to abnormal p. H is slow (hours to days)
Acid Base Imbalances Respiratory Acidosis n n Acidosis is due to hypoventilation Causes: n n COPD (Emphysema, bronchitis) failure of respiratory muscles (ALS, Guillain-Barre) airway obstruction (e. g. , post-op) Metabolic compensation: Kidneys excrete H+/retain HCO 3 - (if problem lasts hours/days)
Acid Base Imbalances Respiratory Alkalosis n n Acidosis is due to hyperventilation Causes n n anxiety (Rx with paper bag) pneumonia pulmonary edema Metabolic compensation: Kidneys excrete HCO 3 - (if problem lasts hours/days)
Acid Base Imbalances Metabolic Acidosis n n Acidosis is due increase in metabolic acids and/or loss of HCO 3 Increased acids due to n n Lost alkali (base) due to: n n n diabetic ketoacidosis renal failure (kidneys cannot excrete H+) poisoning (ASA) severe diarrhea intestinal malabsorption Respiratory compensation: hyperventilation (to blow off CO 2)
Acid Base Imbalances Metabolic Alkalosis n Alkalosis is due to elevated HCO 3 - secondary to loss of acid/H+ or excess alkali intake. n n n Loss of acid Vomiting gastric suction diuretics Minimal respiratory compensation b/c hypoxemia will result and stimulate respirations
ABG Parameters n Pa. O 2 n n Partial pressure of O 2 Normal: 80 - 100 mm. Hg Measures the effectiveness of the lungs in oxygenating the blood. Reflects ability of lungs to diffuse inspired oxygen across the alveolar membrane into the circulating blood Sa. O 2 n n n Oxygen saturation Normal: > 95% % of hgb that is saturated with oxygen.
ABG Parameters n Pa. CO 2 n n Partial Pressure of CO 2 Normal: 35 - 45 mm. Hg Reflects effectiveness of ventilation (movement of air into and out of lungs). HCO 3 n n n – Normal: 22 - 26 m. Eq/l Bicarbonate ion; metabolic parameter. Part of buffer system.
n p. H - Normal 7. 35 - 7. 40 n n n Measures acidity Determined by relative concentrations of CO 2 and HCO 3 Base Excess n n + 2 m. Eq/L Amount of acid or alkali needed to titrate 1 L of fully oxygenated blood to a p. H of 7. 40 when T = 37 & Pa. CO 2 = 40 mm Hg
ABG Interpretation: Assess Oxgenation Step 1 n Look at Pa. O 2 and Sa. O 2 Normal? n Hypoxemic n
ABG Interpretation: Assess Acid-Base Balance Step 2 n Look at p. H Acidotic, alkalotic, or normal? n If normal n High normal? n Low normal? n
ABG Interpretation: Assess Acid-Base Balance Step 3 n Look at Pa. CO 2 n n Is it altered (i. e. increased or decreased)? If altered, consider the direction of the alteration: n n Could it have caused the alteration in p. H? Could it be compensation?
ABG Interpretation: Assess Acid-Base Balance Step 4 n Look at HCO 3 n n Is it altered (i. e. increased or decreased)? If altered, consider the direction of the alteration: n n Could it have caused the alteration in p. H? Could it be compensation?
ABG Interpretation: Assess Acid-Base Balance Step 5 n Decide if the abnormal p. H is caused by the p. CO 2 (respiratory causes) or the HCO 3 (metabolic causes).
ABG Interpretation: Assess Acid-Base Balance Step 6 n Determine if compensation is present n Look at parameter (Pa. CO 2 or HCO 3) that did not cause the p. H disturbance. Has it changed in effort to normalize the p. H? n n If yes, compensation is present. If no, compensation is not present.
- Slides: 20