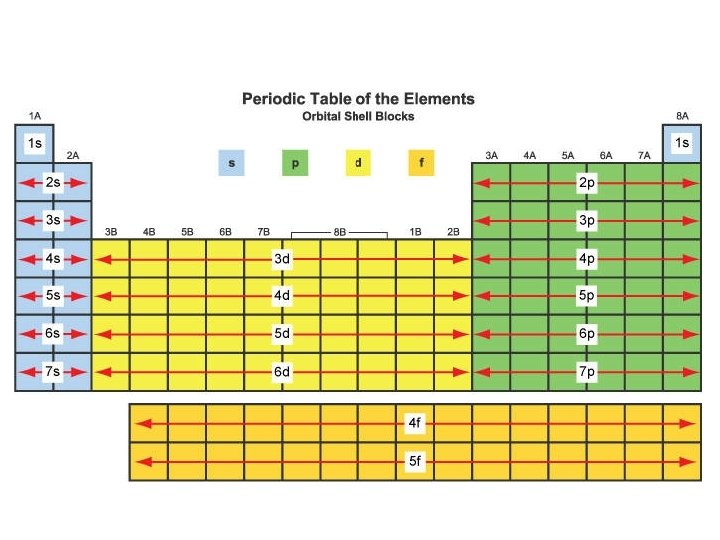
Arrangement of the Periodic Table Vertical columns Called

Arrangement of the Periodic Table

• Vertical columns – Called groups or families – Contain elements with similar chemical and physical properties

• Horizontal rows – Called periods – The length of a period is determined by the number of electrons that can occupy the orbitals filled in that period – Properties vary as you move across a period

• The table is arranged in blocks – s, p, d, and f – Based on the electron configuration of each element – The highest occupied energy level is the period in which the element is found
s-block elements • The s-block elements include groups 1 and 2 as well as hydrogen and helium.

Group 1 • • Alkali metals Silvery in appearance Soft enough to cut with a knife Very reactive – Not found in nature as free elements – Combine vigorously with most non-metals and water – When isolated, they must be store in kerosene to prevent them from reacting with air or moisture

Group 2 • • Alkaline-earth metals Pair of electrons in outermost s orbital Harder, denser, stronger than alkali metals Less reactive than alkali metals, but still too reactive to be found in nature as free elements

Hydrogen and Helium • Hydrogen does not share the same properties as the elements of group 1. It is a unique element with properties that don’t resemble those of any group. • Helium has its outermost energy level full with just 2 electrons. It is placed in group 18 with the other elements with full outer shells (noble gases)
d-block elements • Transition elements (or transition metals) • Good conductors, high luster • Less reactive than group 1 or group 2 metals – Some are so unreactive that they rarely form compounds – Palladium, platinum, and gold are among the least reactive
p-block elements • All have a full s-orbital in outermost energy level, and at least one electron in a p orbital. • Properties vary greatly – Include metals (left and bottom of block) – Nonmetals (right side) – Metalloids (boron, silicon, germanium, arsenic, antimony, and tellurium)

• The metals of the p-block are harder and denser than the alkaline earth metals, but softer and less dense than the transition metals.
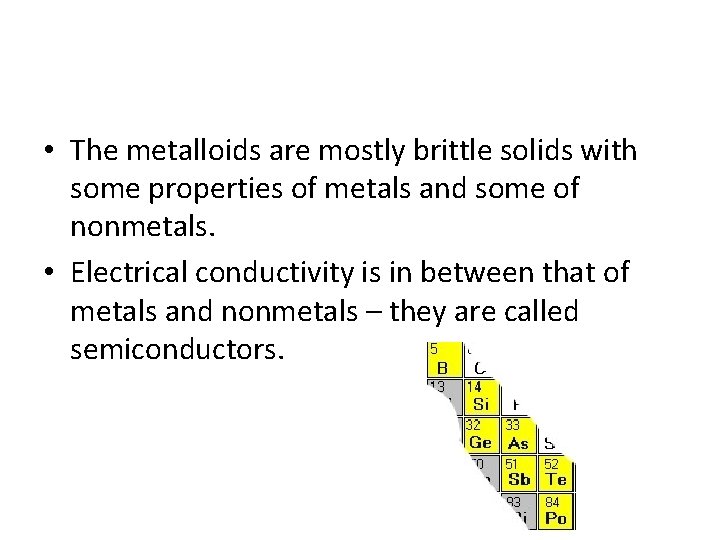
• The metalloids are mostly brittle solids with some properties of metals and some of nonmetals. • Electrical conductivity is in between that of metals and nonmetals – they are called semiconductors.

• All the nonmetals except hydrogen and helium are found in the p block. • They include the halogens and the noble gases.

Halogens • The halogens are group 17. • They are the most reactive nonmetals • React vigorously with most metals to form salts. • At room temperature, fluorine and chlorine are gases, bromine is a reddish liquid, and iodine is a dark-purple solid.
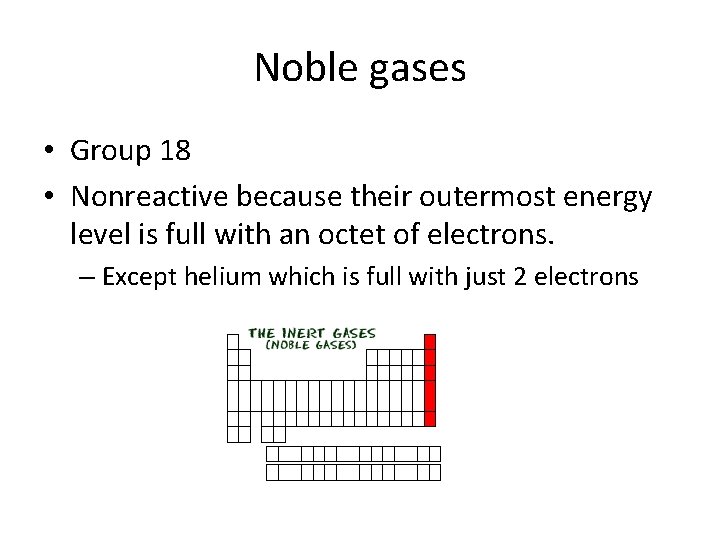
Noble gases • Group 18 • Nonreactive because their outermost energy level is full with an octet of electrons. – Except helium which is full with just 2 electrons

f-block elements • Lanthanides and actinides • Fall between groups 3 and 4, but do NOT belong to any group themselves. • Lanthanides are shiny metals which are similar in reactivity to the alkaline earth metals • Actinides are all radioactive and all but the first 4 are synthetic.
- Slides: 17