Aromaticity of Benzenoid and Nonbenzenoid compounds PARTV By

Aromaticity of Benzenoid and Non-benzenoid compounds PART-V By Dr. Atul Prasad Sikdar Associate Professor Department of Chemistry Mangaldai College : Assam
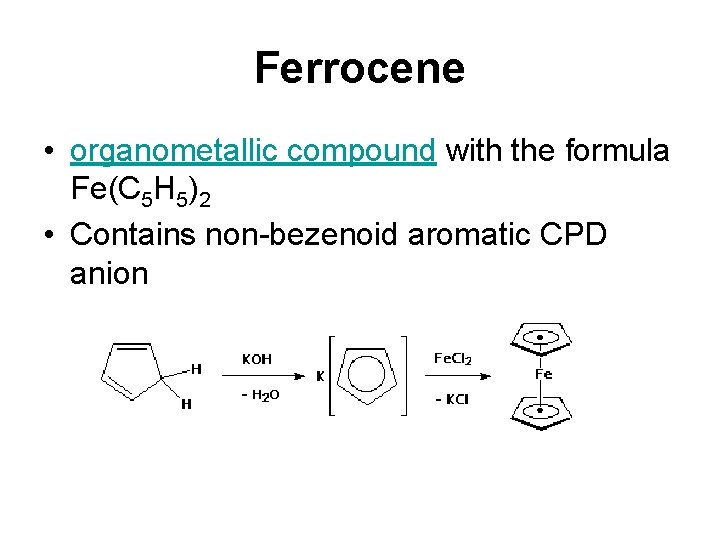
Ferrocene • organometallic compound with the formula Fe(C 5 H 5)2 • Contains non-bezenoid aromatic CPD anion
Ferrocene
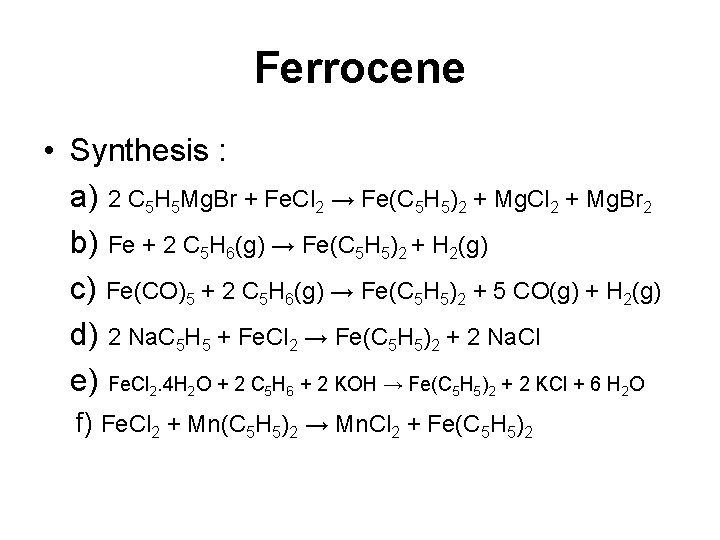
Ferrocene • Synthesis : a) 2 C 5 H 5 Mg. Br + Fe. Cl 2 → Fe(C 5 H 5)2 + Mg. Cl 2 + Mg. Br 2 b) Fe + 2 C 5 H 6(g) → Fe(C 5 H 5)2 + H 2(g) c) Fe(CO)5 + 2 C 5 H 6(g) → Fe(C 5 H 5)2 + 5 CO(g) + H 2(g) d) 2 Na. C 5 H 5 + Fe. Cl 2 → Fe(C 5 H 5)2 + 2 Na. Cl e) Fe. Cl 2. 4 H 2 O + 2 C 5 H 6 + 2 KOH → Fe(C 5 H 5)2 + 2 KCl + 6 H 2 O f) Fe. Cl 2 + Mn(C 5 H 5)2 → Mn. Cl 2 + Fe(C 5 H 5)2
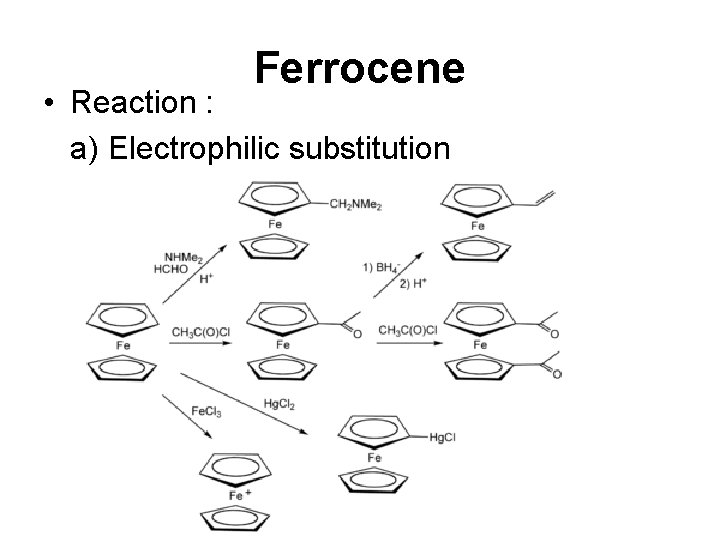
Ferrocene • Reaction : a) Electrophilic substitution
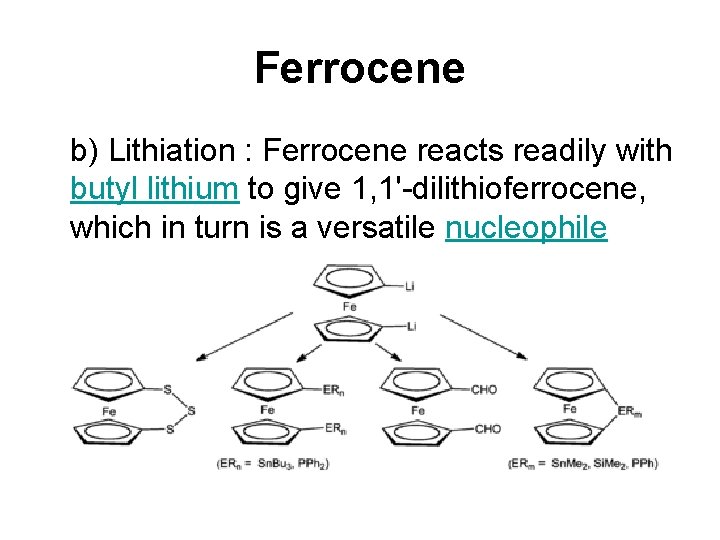
Ferrocene b) Lithiation : Ferrocene reacts readily with butyl lithium to give 1, 1'-dilithioferrocene, which in turn is a versatile nucleophile
Azulene • Azulene is an organic compound an isomer of naphthalene • Azulene is usually viewed as resulting from fusion of cyclopentadiene and cycloheptatriene rings
Azulene • It exhibits aromatic properties • the peripheral bonds have similar lengths and it undergoes Friedel-Crafts-like substitutions • The stability gain from aromaticity is estimated to be half that of naphthalene. • Its dipole moment is 1. 08 D (Naphthalene- 0 D)
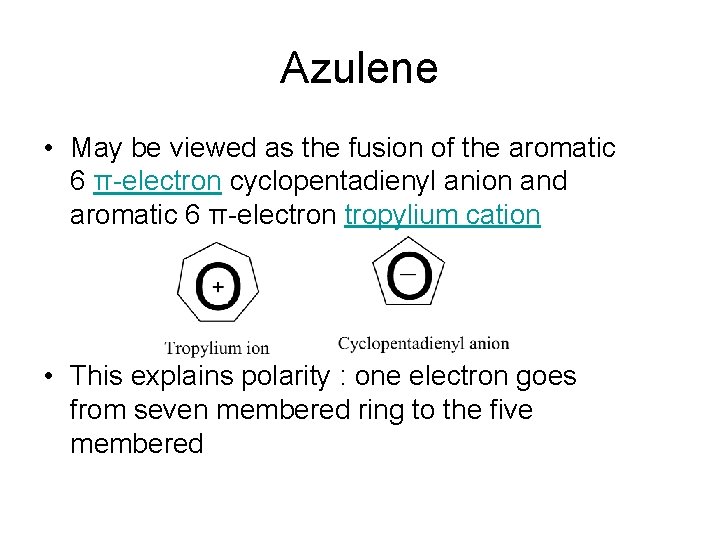
Azulene • May be viewed as the fusion of the aromatic 6 π-electron cyclopentadienyl anion and aromatic 6 π-electron tropylium cation • This explains polarity : one electron goes from seven membered ring to the five membered

Azulene • seven-membered ring is electrophilic and the five-membered ring is nucleophilic. • The dipolar nature of the ground state is reflected in its deep colour, which is unusual for small unsaturated aromatic compounds. • exhibits fluorescence from an upperexcited state (S 2 → S 0)

Azulene • The blue color of the mushroom Lactarius indigo is due to the azulene derivative (7 isopropenyl-4 -methylazulen-1 -yl)methyl stearate
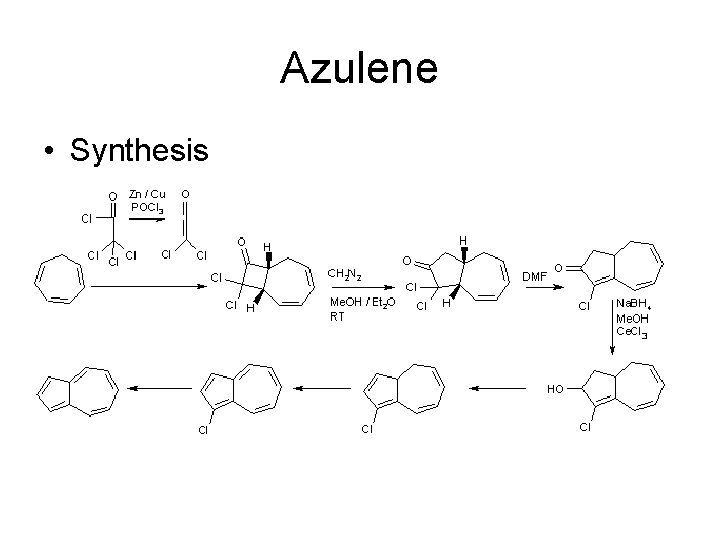
Azulene • Synthesis
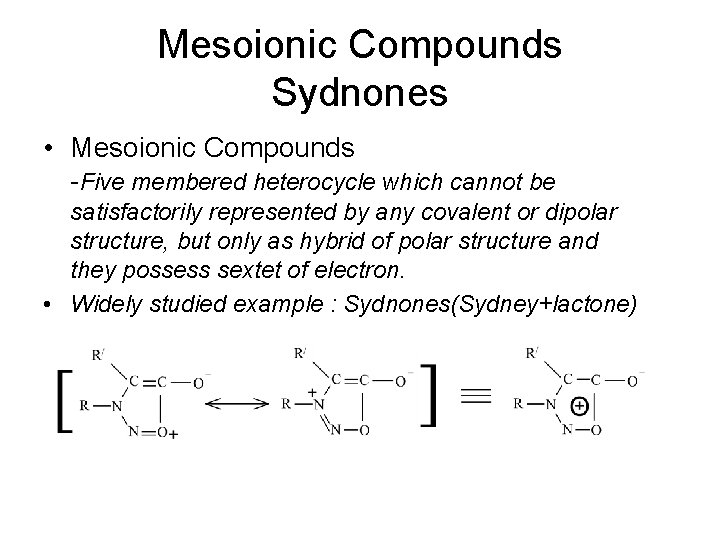
Mesoionic Compounds Sydnones • Mesoionic Compounds -Five membered heterocycle which cannot be satisfactorily represented by any covalent or dipolar structure, but only as hybrid of polar structure and they possess sextet of electron. • Widely studied example : Sydnones(Sydney+lactone)

Sydnones • Synthesis : From primary amine
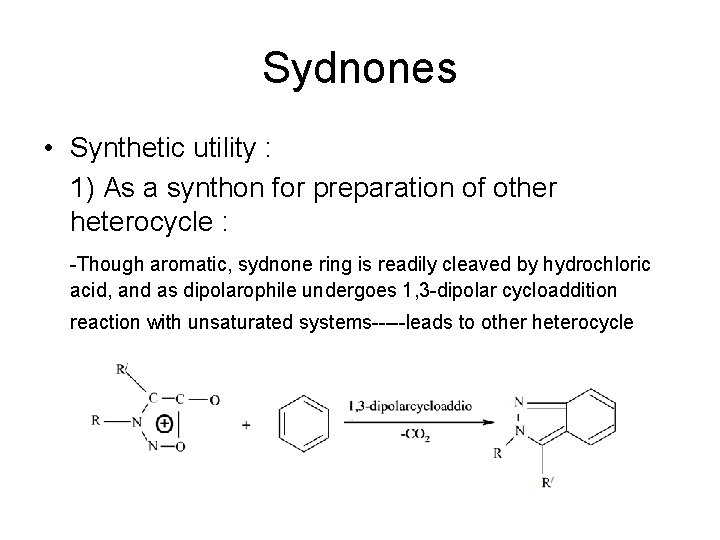
Sydnones • Synthetic utility : 1) As a synthon for preparation of other heterocycle : -Though aromatic, sydnone ring is readily cleaved by hydrochloric acid, and as dipolarophile undergoes 1, 3 -dipolar cycloaddition reaction with unsaturated systems-----leads to other heterocycle
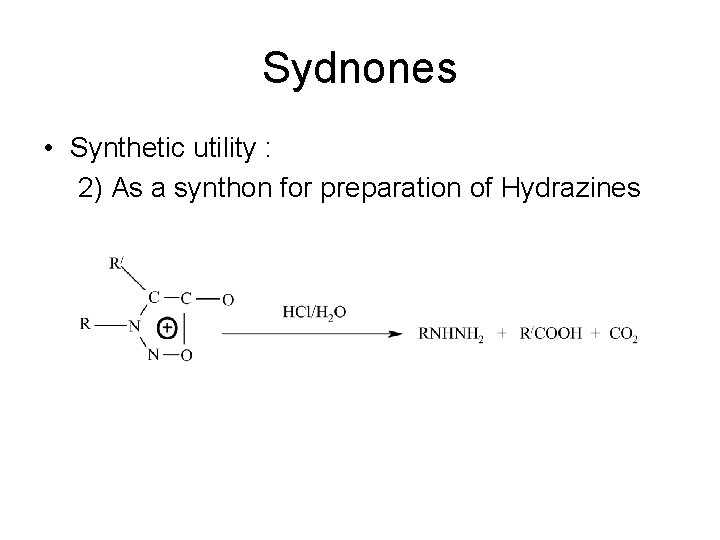
Sydnones • Synthetic utility : 2) As a synthon for preparation of Hydrazines
Spectroscopy of Aromatic Compounds Aromatic compounds can be identified by: – Infrared (IR) Spectroscopy – Ultraviolet (UV) Spectroscopy – Nuclear Magnetic Resonance (NMR) Spectroscopy
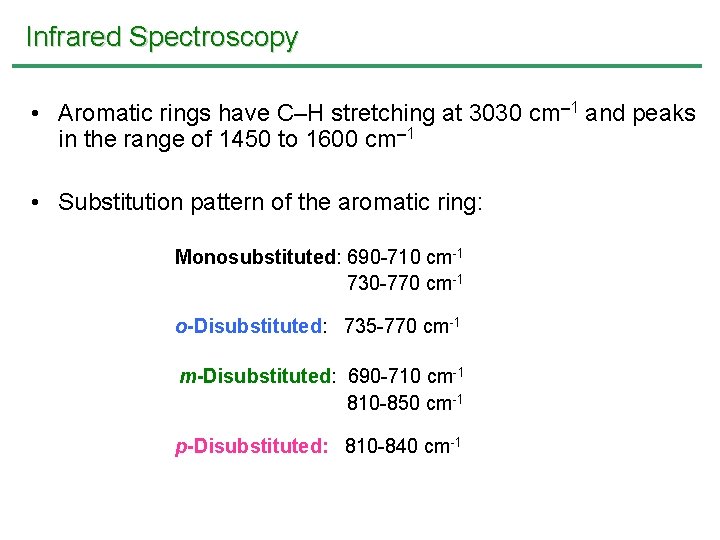
Infrared Spectroscopy • Aromatic rings have C–H stretching at 3030 cm 1 and peaks in the range of 1450 to 1600 cm 1 • Substitution pattern of the aromatic ring: Monosubstituted: 690 -710 cm-1 730 -770 cm-1 o-Disubstituted: 735 -770 cm-1 m-Disubstituted: 690 -710 cm-1 810 -850 cm-1 p-Disubstituted: 810 -840 cm-1
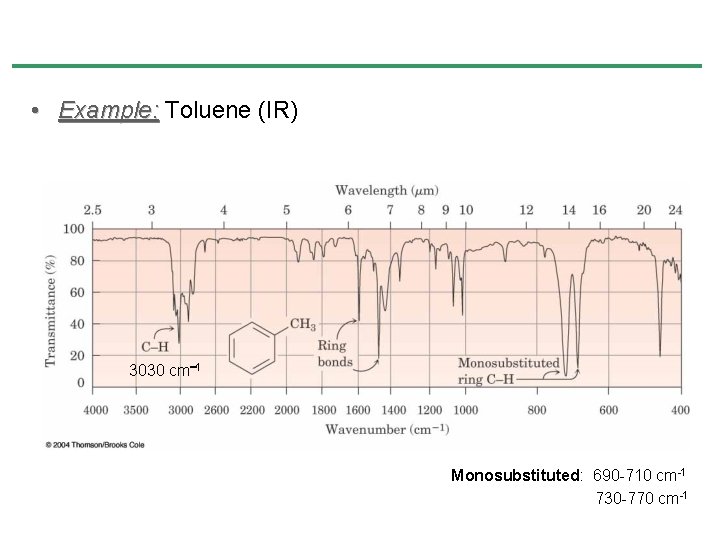
• Example: Toluene (IR) 3030 cm 1 Monosubstituted: 690 -710 cm-1 730 -770 cm-1
Ultraviolet Spectroscopy • Aromatic rings have peaks near 205 nm and a less intense peak in 255 -275 nm range – Aromatic compounds are detectable by UV spectroscopy since they have a conjugated electron system
Nuclear Magnetic Resonance Spectroscopy 1 H NMR: – Aromatic H’s are strongly deshielded by ring and absorb between 6. 5 and 8. 0 – Peak pattern is characteristic positions of substituents
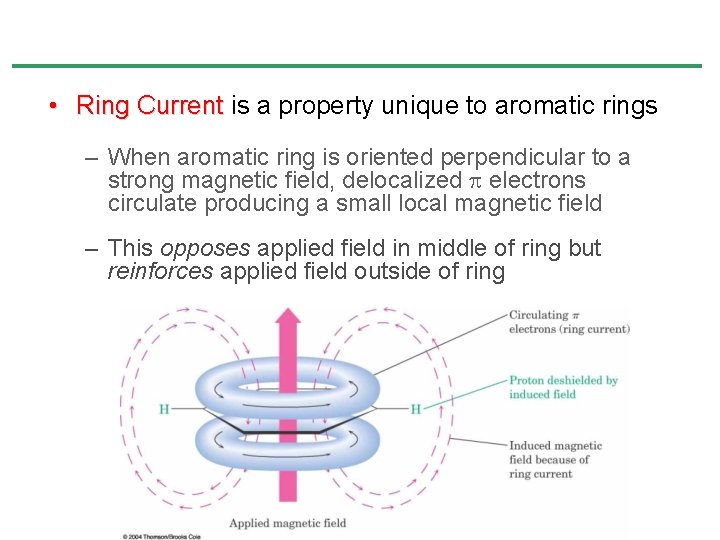
• Ring Current is a property unique to aromatic rings – When aromatic ring is oriented perpendicular to a strong magnetic field, delocalized electrons circulate producing a small local magnetic field – This opposes applied field in middle of ring but reinforces applied field outside of ring
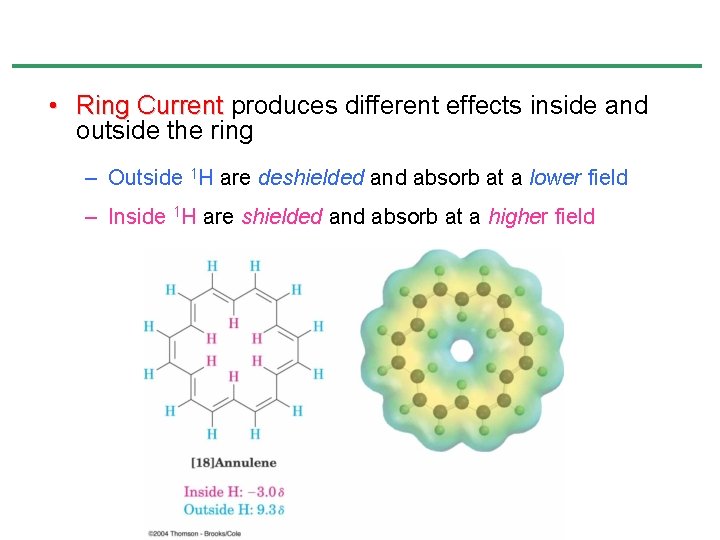
• Ring Current produces different effects inside and outside the ring – Outside 1 H are deshielded and absorb at a lower field – Inside 1 H are shielded and absorb at a higher field
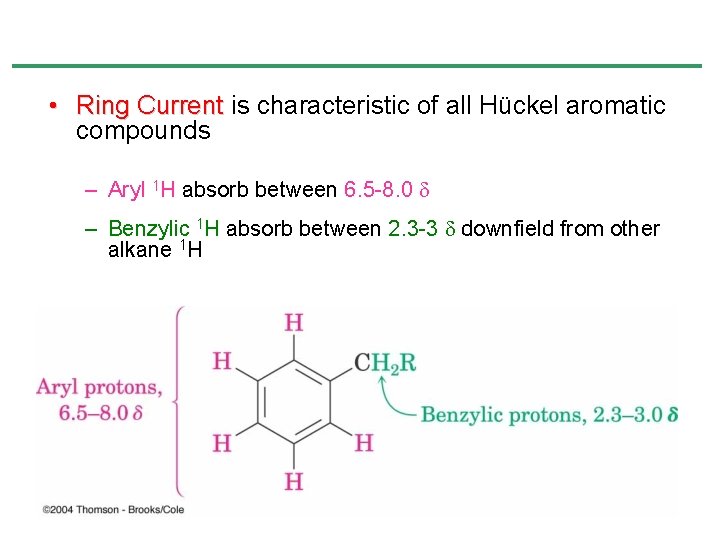
• Ring Current is characteristic of all Hückel aromatic compounds – Aryl 1 H absorb between 6. 5 -8. 0 – Benzylic 1 H absorb between 2. 3 -3 downfield from other alkane 1 H
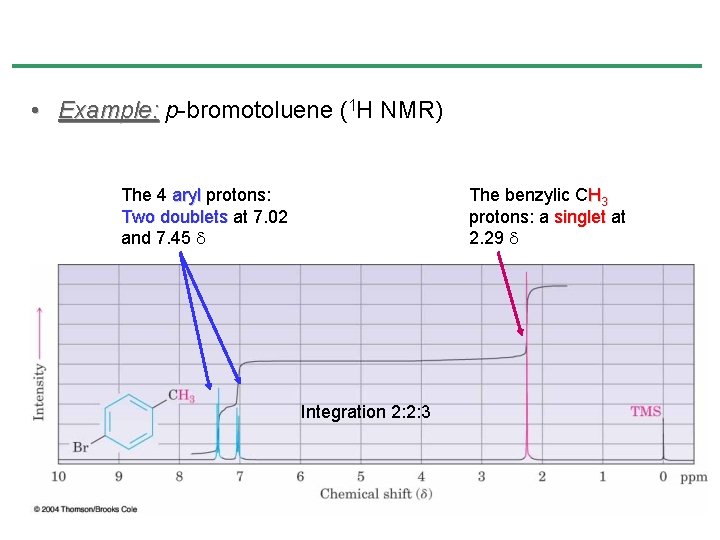
• Example: p-bromotoluene (1 H NMR) The 4 aryl protons: Two doublets at 7. 02 and 7. 45 The benzylic CH 3 protons: a singlet at 2. 29 Integration 2: 2: 3
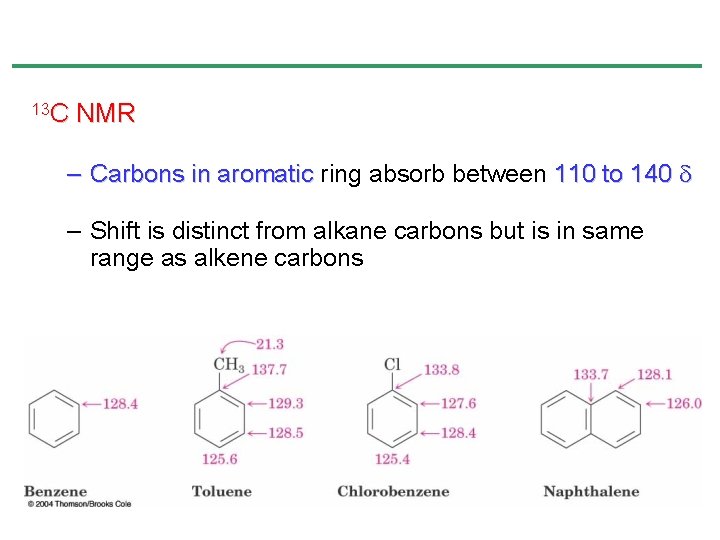
13 C NMR – Carbons in aromatic ring absorb between 110 to 140 – Shift is distinct from alkane carbons but is in same range as alkene carbons
Multiple Substituent Effects in Electrophilic Aromatic Substitution(EAS)
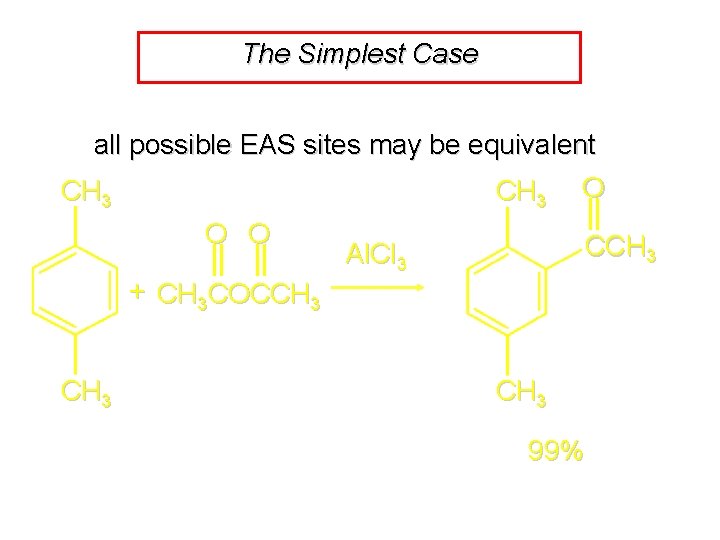
The Simplest Case all possible EAS sites may be equivalent CH 3 O O O CCH 3 Al. Cl 3 + CH 3 COCCH 3 99%
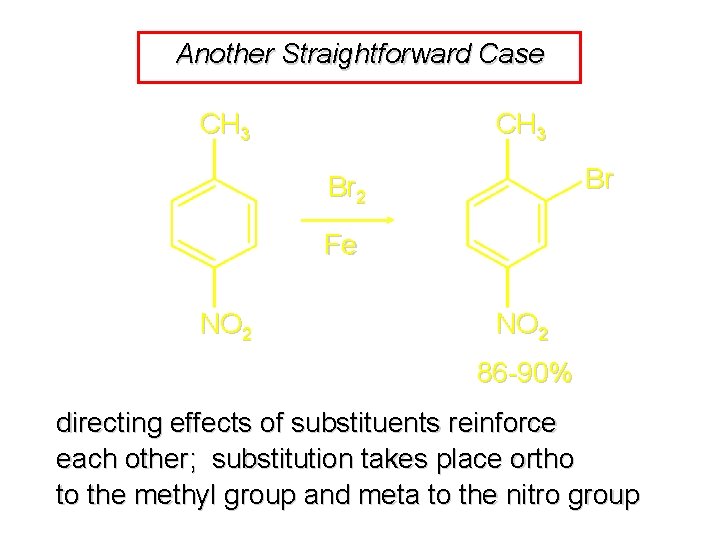
Another Straightforward Case CH 3 Br Br 2 Fe NO 2 86 -90% directing effects of substituents reinforce each other; substitution takes place ortho to the methyl group and meta to the nitro group

Generalization regioselectivity is controlled by the most activating substituent
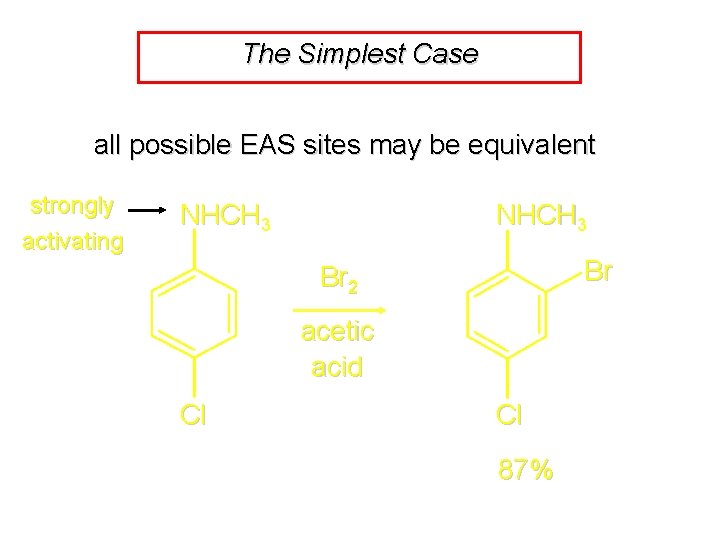
The Simplest Case all possible EAS sites may be equivalent strongly activating NHCH 3 Br Br 2 acetic acid Cl Cl 87%
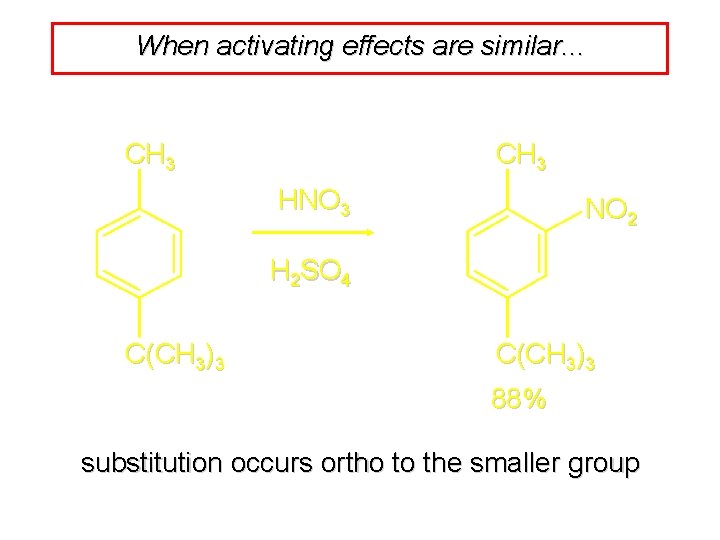
When activating effects are similar. . . CH 3 HNO 3 NO 2 H 2 SO 4 C(CH 3)3 88% substitution occurs ortho to the smaller group
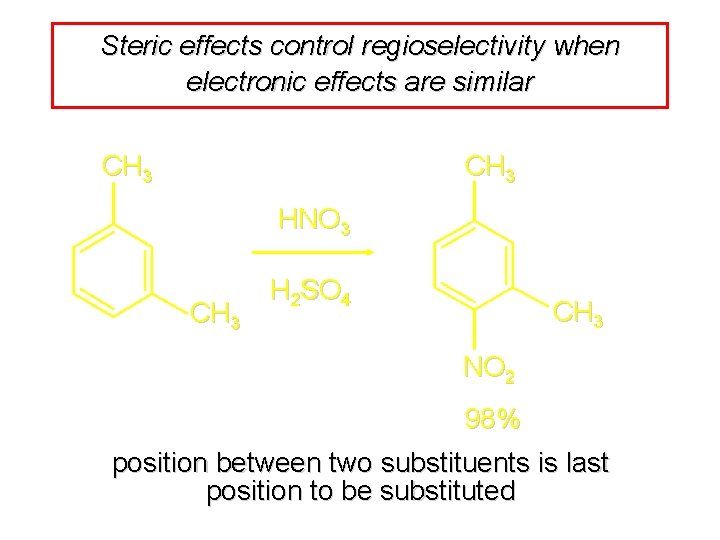
Steric effects control regioselectivity when electronic effects are similar CH 3 HNO 3 CH 3 H 2 SO 4 CH 3 NO 2 98% position between two substituents is last position to be substituted
Regioselective Synthesis of Disubstituted Aromatic Compounds Factors to Consider order of introduction of substituents to ensure correct orientation
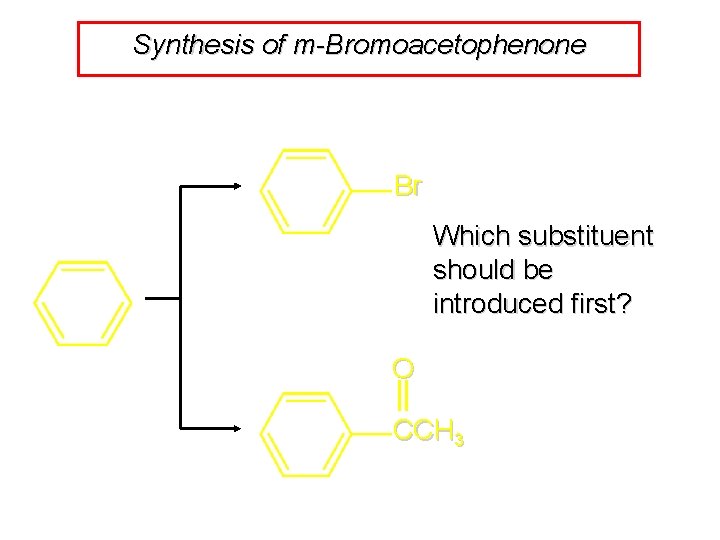
Synthesis of m-Bromoacetophenone Br Which substituent should be introduced first? O CCH 3
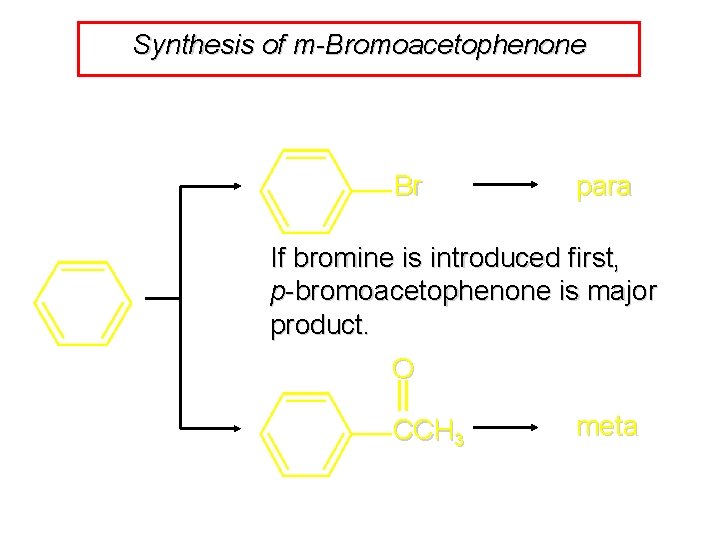
Synthesis of m-Bromoacetophenone Br para If bromine is introduced first, p-bromoacetophenone is major product. O CCH 3 meta
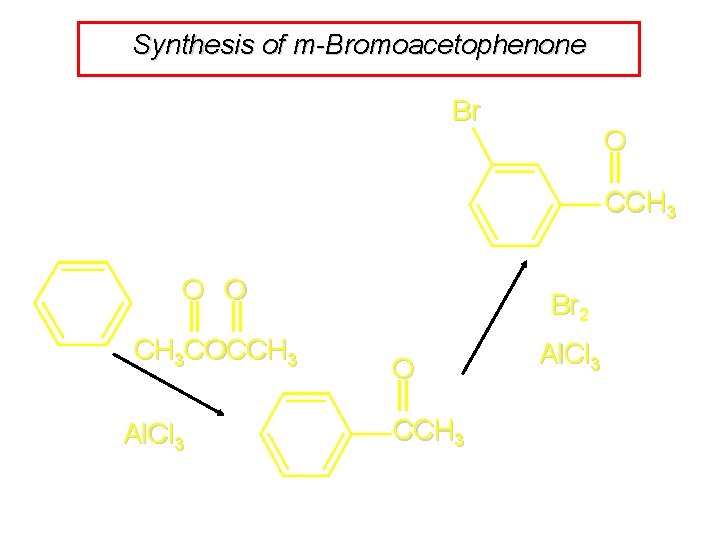
Synthesis of m-Bromoacetophenone Br O CCH 3 O O CH 3 COCCH 3 Al. Cl 3 Br 2 O CCH 3 Al. Cl 3

Factors to Consider order of introduction of substituents to ensure correct orientation Friedel-Crafts reactions (alkylation, acylation) cannot be carried out on strongly deactivated aromatics
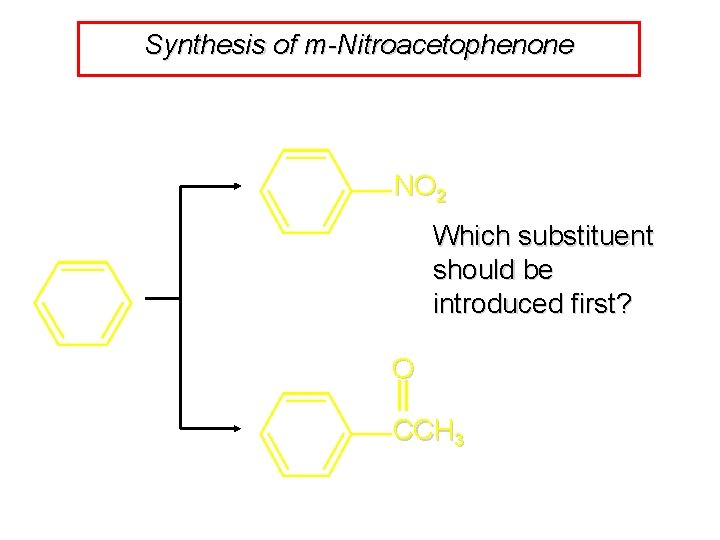
Synthesis of m-Nitroacetophenone NO 2 Which substituent should be introduced first? O CCH 3
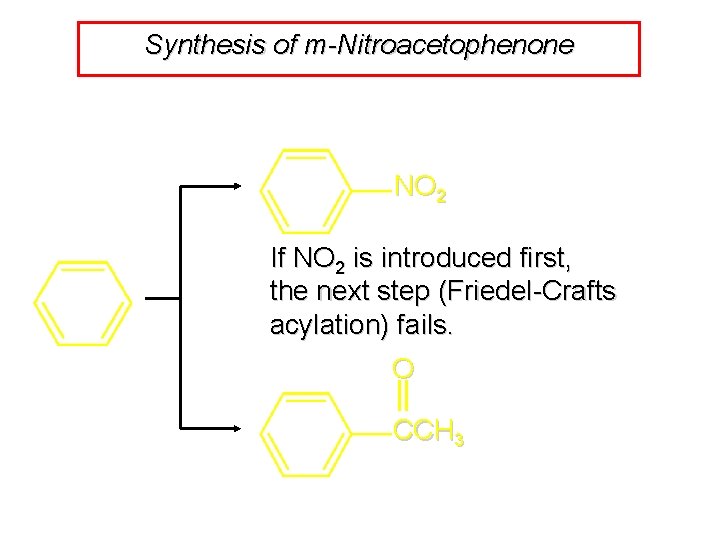
Synthesis of m-Nitroacetophenone NO 2 If NO 2 is introduced first, the next step (Friedel-Crafts acylation) fails. O CCH 3
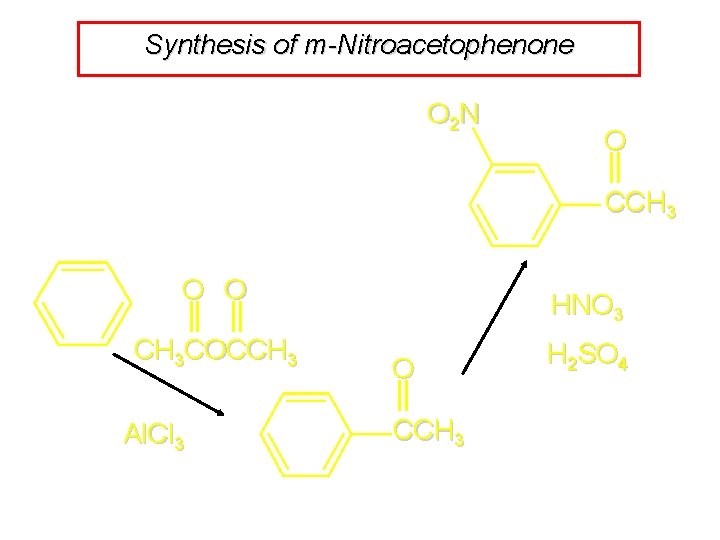
Synthesis of m-Nitroacetophenone O 2 N O CCH 3 O O CH 3 COCCH 3 Al. Cl 3 HNO 3 O CCH 3 H 2 SO 4
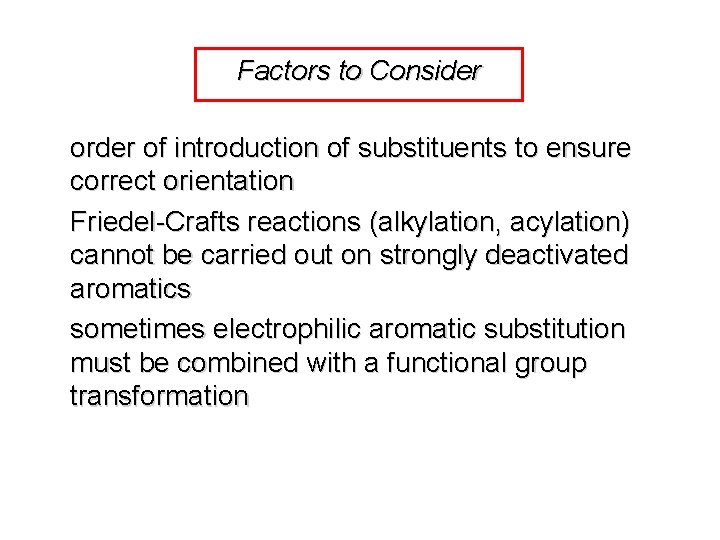
Factors to Consider order of introduction of substituents to ensure correct orientation Friedel-Crafts reactions (alkylation, acylation) cannot be carried out on strongly deactivated aromatics sometimes electrophilic aromatic substitution must be combined with a functional group transformation
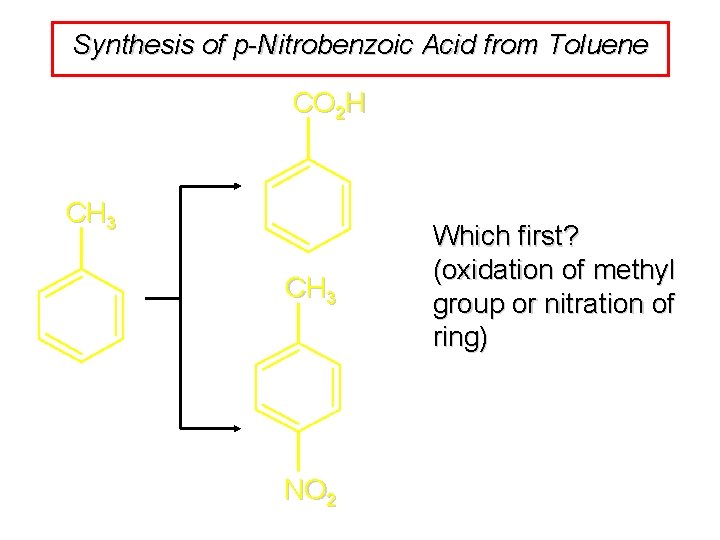
Synthesis of p-Nitrobenzoic Acid from Toluene CO 2 H CH 3 NO 2 Which first? (oxidation of methyl group or nitration of ring)
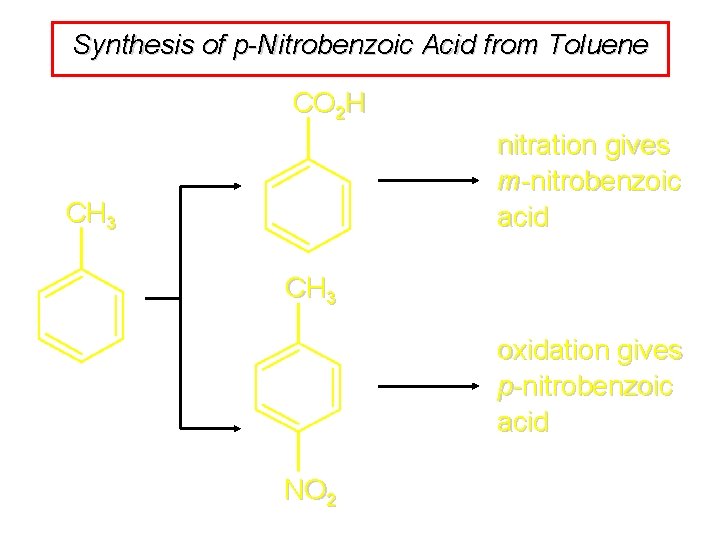
Synthesis of p-Nitrobenzoic Acid from Toluene CO 2 H nitration gives m-nitrobenzoic acid CH 3 oxidation gives p-nitrobenzoic acid NO 2
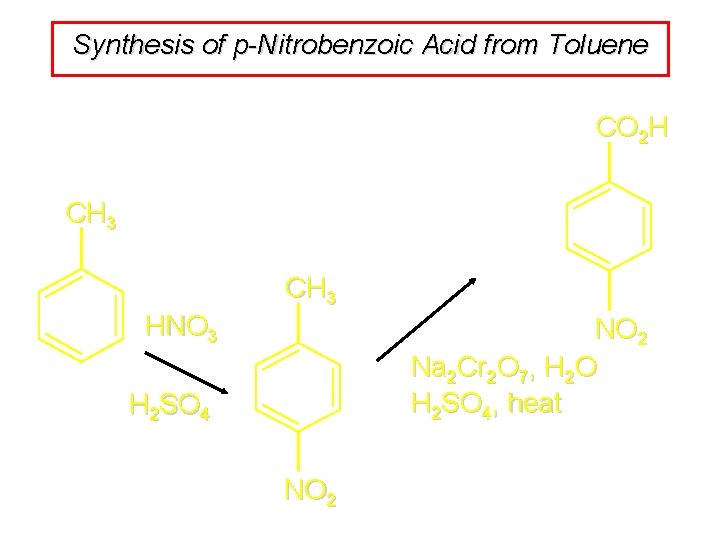
Synthesis of p-Nitrobenzoic Acid from Toluene CO 2 H CH 3 HNO 3 NO 2 Na 2 Cr 2 O 7, H 2 O H 2 SO 4, heat H 2 SO 4 NO 2
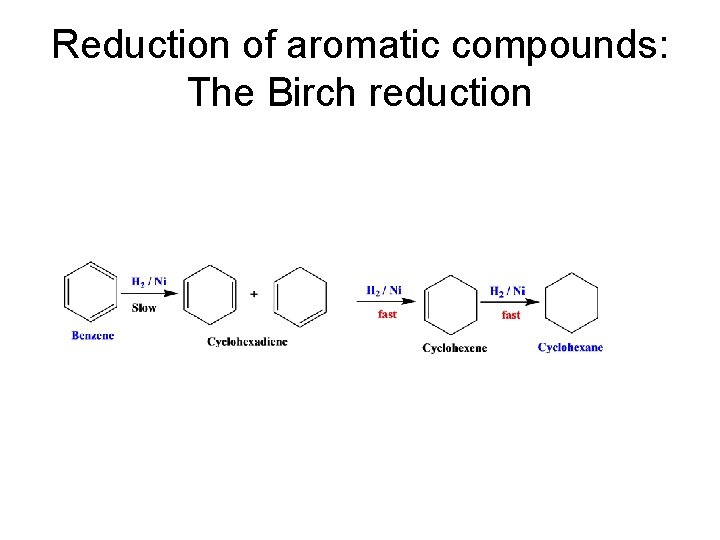
Reduction of aromatic compounds: The Birch reduction
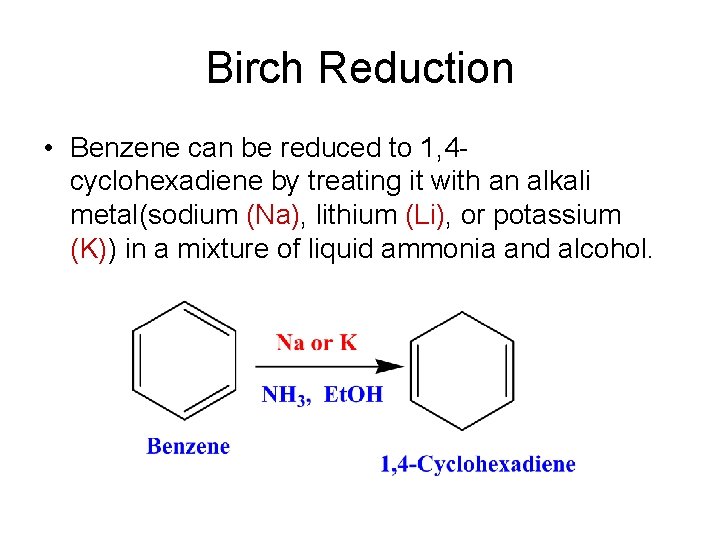
Birch Reduction • Benzene can be reduced to 1, 4 cyclohexadiene by treating it with an alkali metal(sodium (Na), lithium (Li), or potassium (K)) in a mixture of liquid ammonia and alcohol.
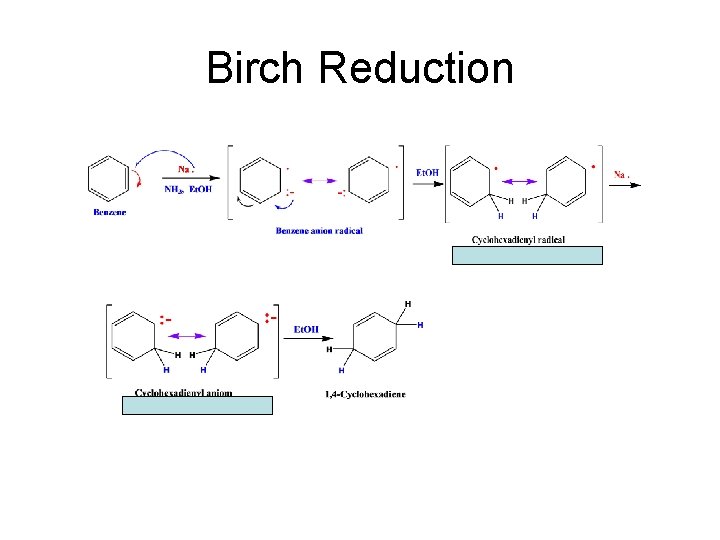
Birch Reduction
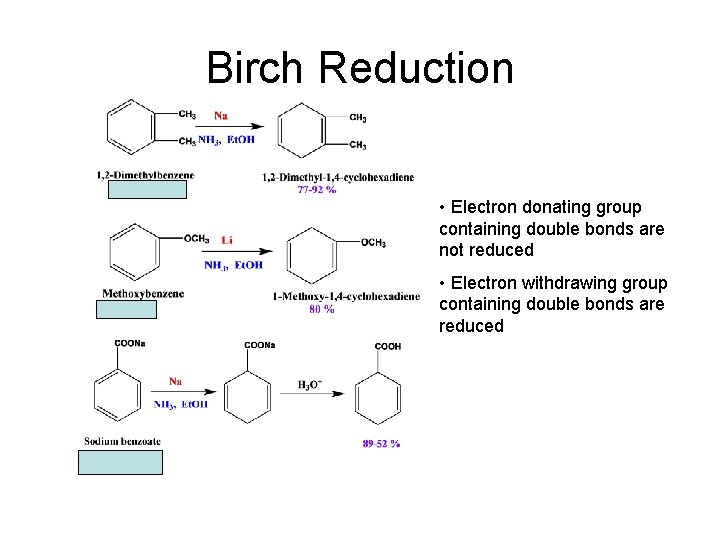
Birch Reduction • Electron donating group containing double bonds are not reduced • Electron withdrawing group containing double bonds are reduced

- Slides: 51