Aromaticity of Benzenoid and Nonbenzenoid compounds PARTII By

Aromaticity of Benzenoid and Non-benzenoid compounds PART-II By Dr. Atul Prasad Sikdar Associate Professor Department of Chemistry Mangaldai College : Assam
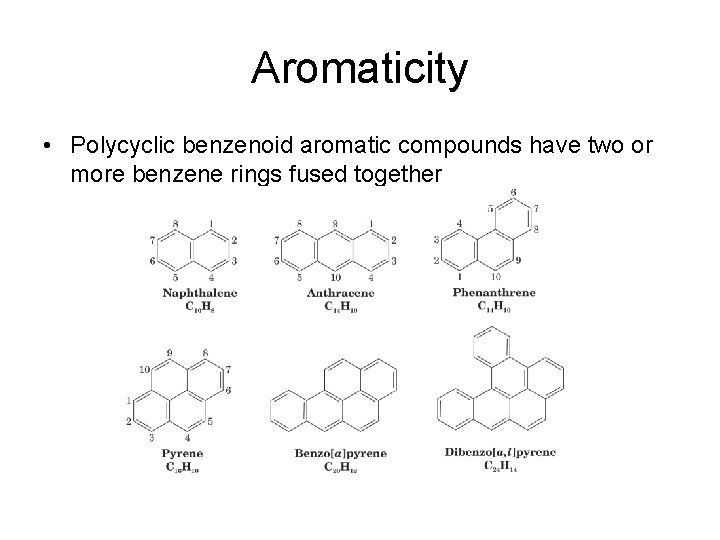
Aromaticity • Polycyclic benzenoid aromatic compounds have two or more benzene rings fused together
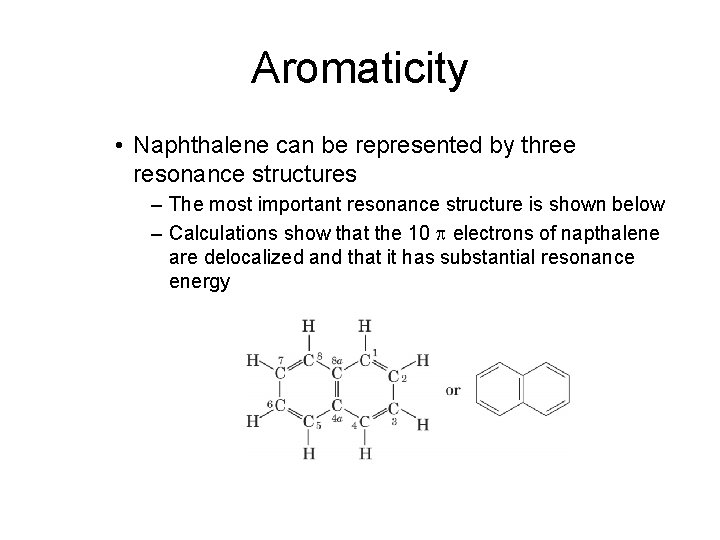
Aromaticity • Naphthalene can be represented by three resonance structures – The most important resonance structure is shown below – Calculations show that the 10 p electrons of napthalene are delocalized and that it has substantial resonance energy

Aromaticity • Pyrene has 16 p electrons, a non-Huckel number, yet is known to be aromatic – Ignoring the central double bond, the periphery of pyrene has 14 p electrons, a Huckel number, and on this basis it resembles the aromatic [14]annulene
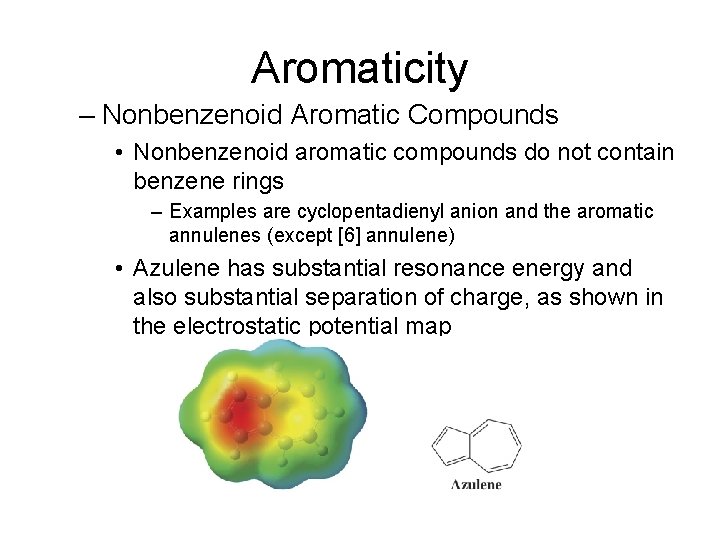
Aromaticity – Nonbenzenoid Aromatic Compounds • Nonbenzenoid aromatic compounds do not contain benzene rings – Examples are cyclopentadienyl anion and the aromatic annulenes (except [6] annulene) • Azulene has substantial resonance energy and also substantial separation of charge, as shown in the electrostatic potential map
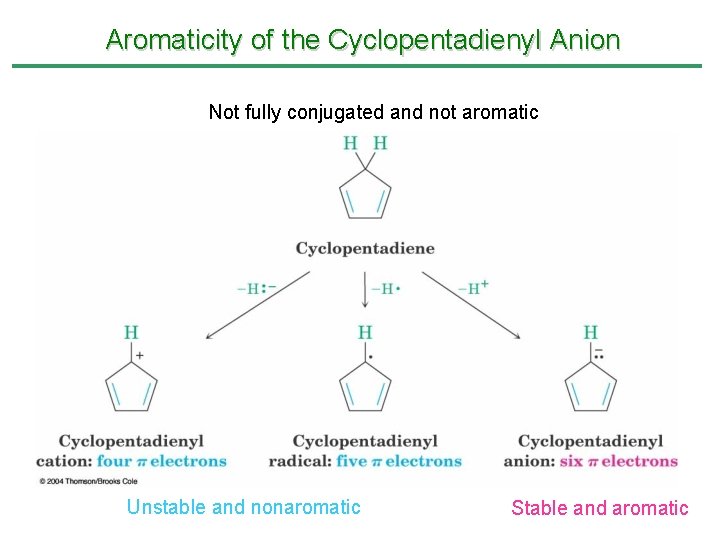
Aromaticity of the Cyclopentadienyl Anion Not fully conjugated and not aromatic Unstable and nonaromatic Stable and aromatic
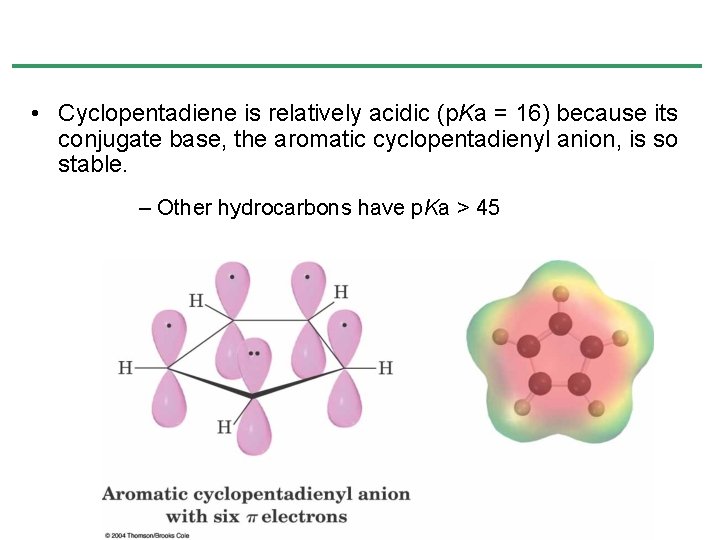
• Cyclopentadiene is relatively acidic (p. Ka = 16) because its conjugate base, the aromatic cyclopentadienyl anion, is so stable. – Other hydrocarbons have p. Ka > 45
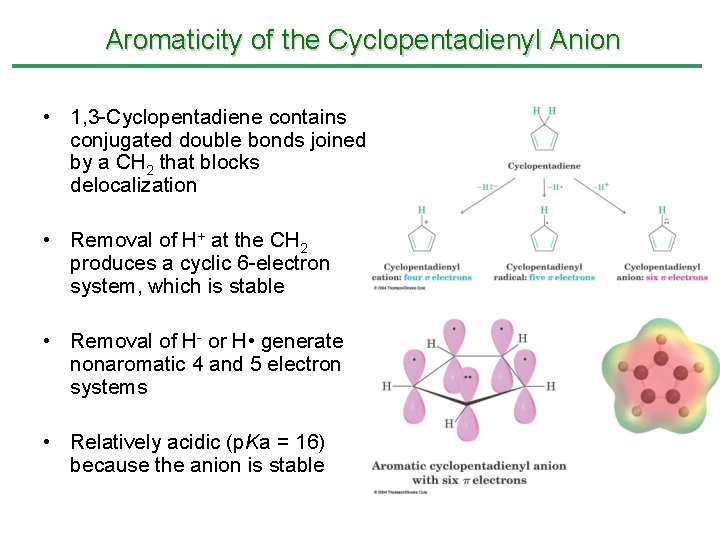
Aromaticity of the Cyclopentadienyl Anion • 1, 3 -Cyclopentadiene contains conjugated double bonds joined by a CH 2 that blocks delocalization • Removal of H+ at the CH 2 produces a cyclic 6 -electron system, which is stable • Removal of H- or H • generate nonaromatic 4 and 5 electron systems • Relatively acidic (p. Ka = 16) because the anion is stable
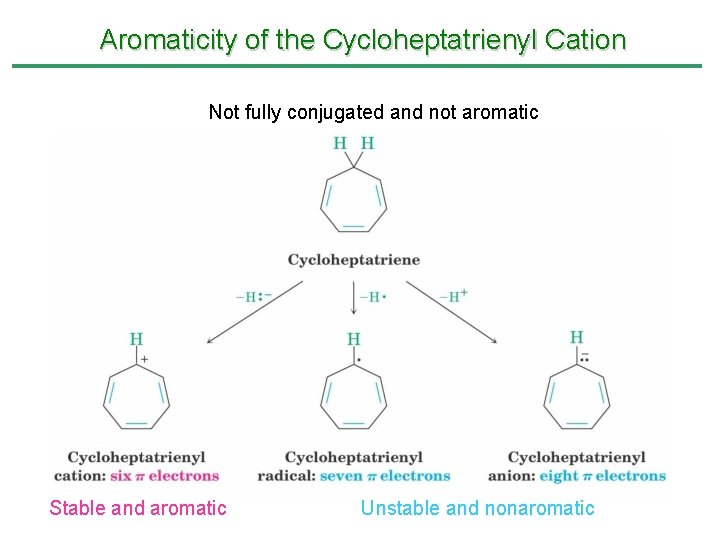
Aromaticity of the Cycloheptatrienyl Cation Not fully conjugated and not aromatic Stable and aromatic Unstable and nonaromatic
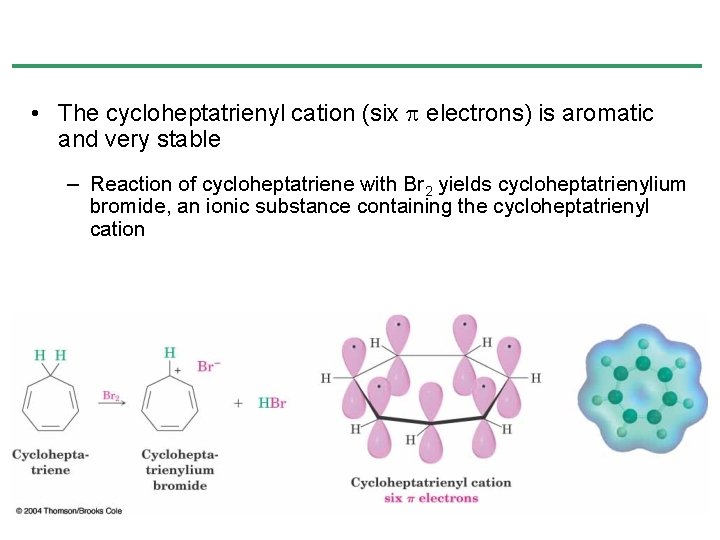
• The cycloheptatrienyl cation (six p electrons) is aromatic and very stable – Reaction of cycloheptatriene with Br 2 yields cycloheptatrienylium bromide, an ionic substance containing the cycloheptatrienyl cation
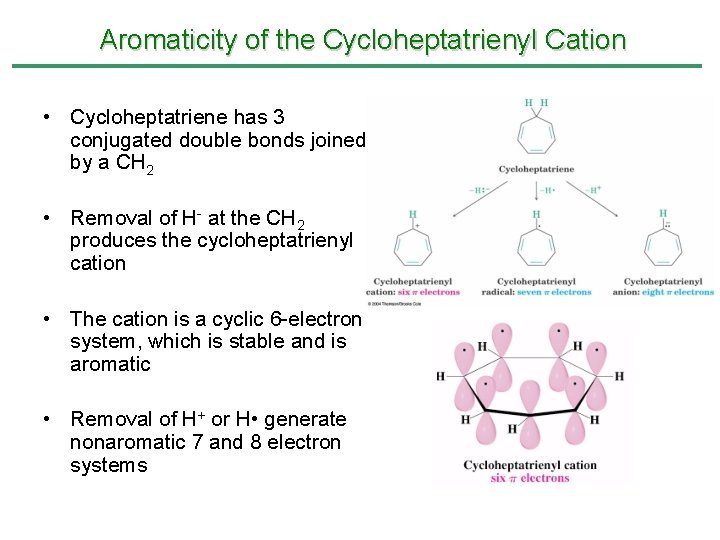
Aromaticity of the Cycloheptatrienyl Cation • Cycloheptatriene has 3 conjugated double bonds joined by a CH 2 • Removal of H- at the CH 2 produces the cycloheptatrienyl cation • The cation is a cyclic 6 -electron system, which is stable and is aromatic • Removal of H+ or H • generate nonaromatic 7 and 8 electron systems

- Slides: 12