AROMATIC HYDROCARBONS Section 1 3 SUMMARY BENZENE RING

AROMATIC HYDROCARBONS Section 1. 3
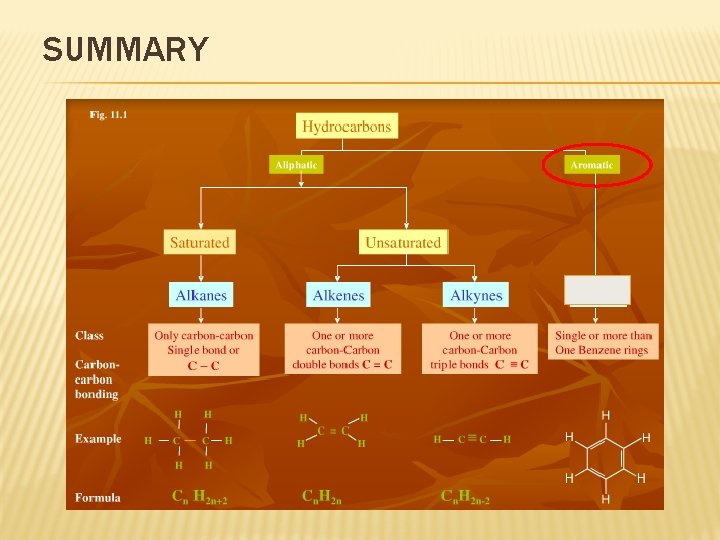
SUMMARY
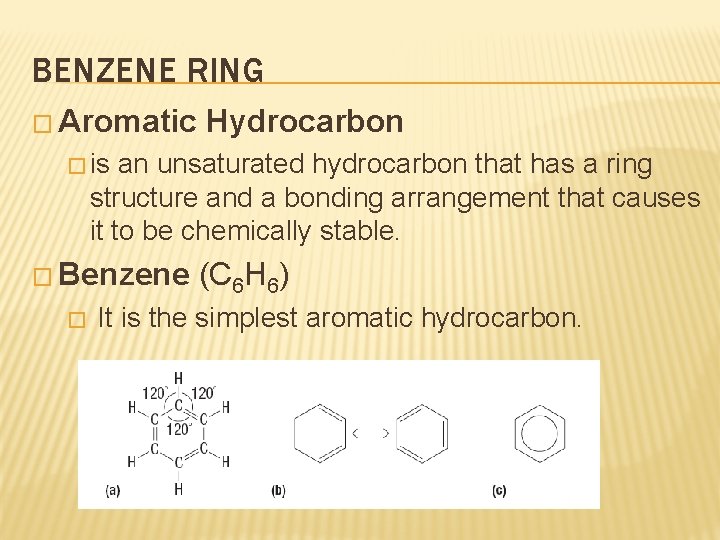
BENZENE RING � Aromatic Hydrocarbon � is an unsaturated hydrocarbon that has a ring structure and a bonding arrangement that causes it to be chemically stable. � Benzene � (C 6 H 6) It is the simplest aromatic hydrocarbon.
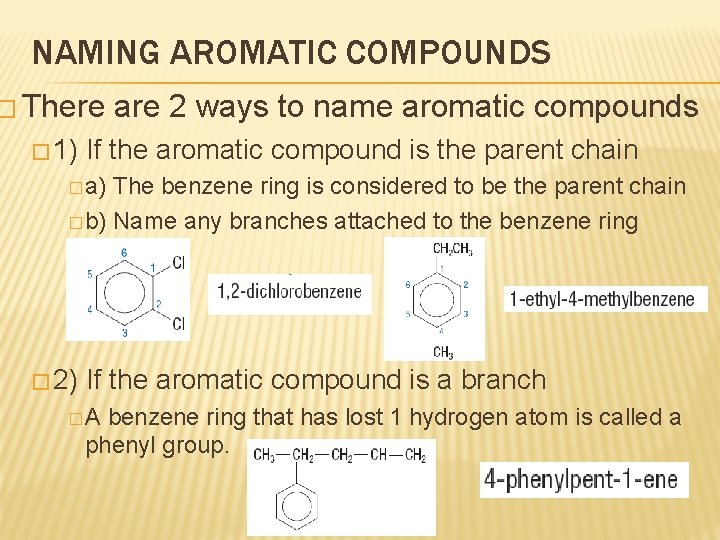
NAMING AROMATIC COMPOUNDS � There � 1) are 2 ways to name aromatic compounds If the aromatic compound is the parent chain � a) The benzene ring is considered to be the parent chain � b) Name any branches attached to the benzene ring � 2) If the aromatic compound is a branch �A benzene ring that has lost 1 hydrogen atom is called a phenyl group.
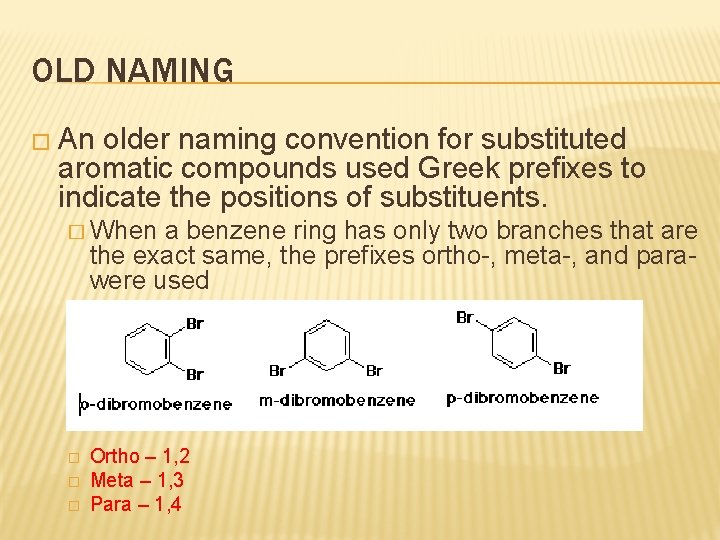
OLD NAMING � An older naming convention for substituted aromatic compounds used Greek prefixes to indicate the positions of substituents. � When a benzene ring has only two branches that are the exact same, the prefixes ortho-, meta-, and parawere used � � � Ortho – 1, 2 Meta – 1, 3 Para – 1, 4
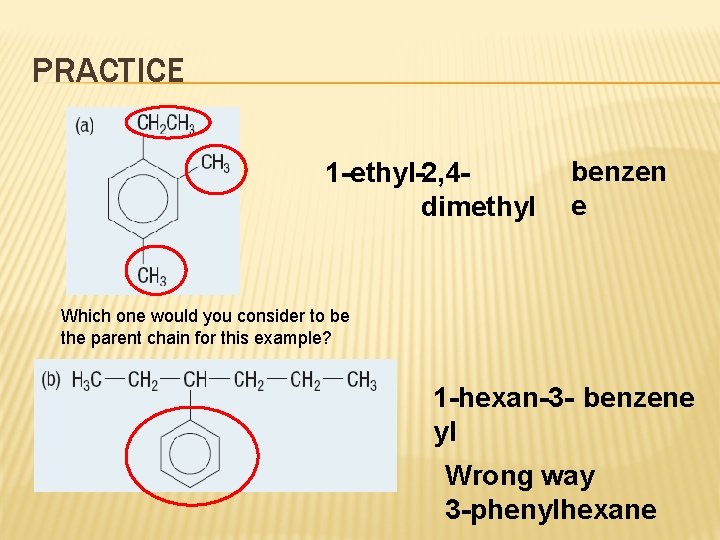
PRACTICE 1 -ethyl-2, 4 dimethyl benzen e Which one would you consider to be the parent chain for this example? 1 -hexan-3 - benzene yl Wrong way 3 -phenylhexane
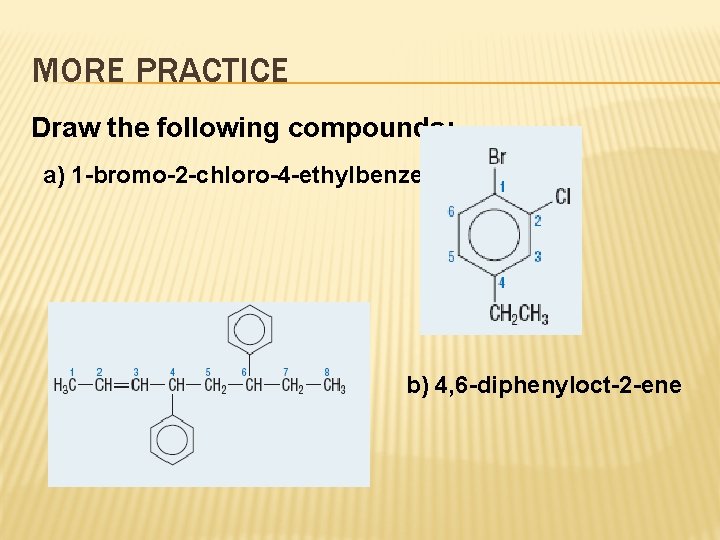
MORE PRACTICE Draw the following compounds: a) 1 -bromo-2 -chloro-4 -ethylbenzene b) 4, 6 -diphenyloct-2 -ene

PROPERTIES OF AROMATIC HYDROCARBONS � Many aromatic hydrocarbons are liquids at room temperature, while others are crystalline solids. � Their symmetrical structure causes them to be non-polar, so they are generally insoluble in water. (Like dissolves in like)
- Slides: 8