Aromatic compounds Organic compound that contains a benzene

Aromatic compounds • Organic compound that contains a benzene ring in its molecule is known as an aromatic compounds. • Sometimes called arenes. • Molecular formula: C 6 H 6 • Represented as a regular hexagon containing an inscribed circle.

Structure of Benzene • Can be represented in two abbreviated ways. • The corner of each hexagon represents a carbon and a hydrogen atom.

Kekulé Structure of Benzene Molecular formula is C 6 H 6 All the hydrogen atoms are equivalent Each carbon atom must have four covalent bonds.

Resonance Structure • Resonance theory: the structure of benzene is a resonance hybrid structure of two Kekulé cononical forms. • The hybrid structure is often represented by a hexagon containing an inscribed circle.

• Hexagonal ring – 6 carbon-carbon bonds are equal. • Circle – delocalised electrons of the benzene ring

Naming Aromatic Compounds

• A substituted benzene is derived by replacing one or more of benzene’s hydrogen atoms with an atom or group of atoms. • A monosubstituted benzene has the formula C 6 H 5 G where G is the group that replaces a hydrogen atom. • All hydrogens in benzene are equivalent. • It does not matter which hydrogen is replaced by G.

Monosubstituted Benzenes

• Some monosubstituted benzenes are named by adding the name of the substituent group as a prefix to the word benzene. • The name is written as one word. nitro group nitrobenzene ethyl group ethylbenzene

• Certain monosubstituted benzenes have special names. • These are parent names for further substituted compounds. hydroxy group methyl group toluene phenol

carboxyl group amino group benzoic acid aniline

• Disubstituted Benzenes

• Three isomers are possible when two substituents replace hydrogen in a benzene molecule. • The prefixes ortho-, meta- and para- (o-, m- and p-) are used to name these disubstituted benzenes.
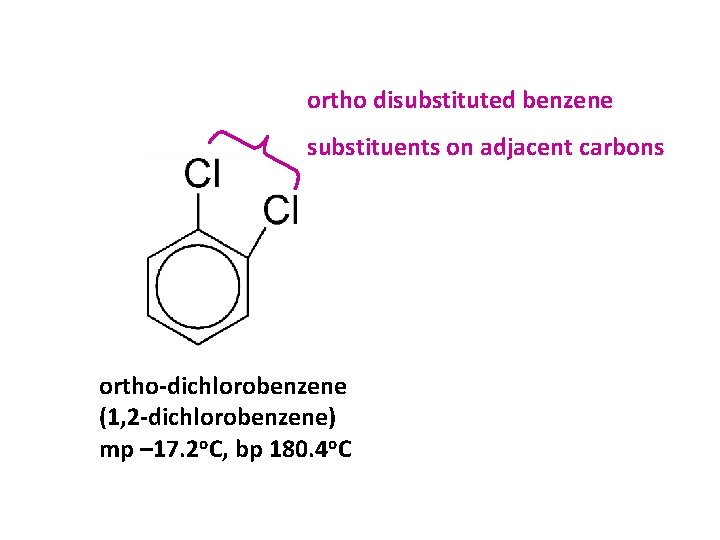
ortho disubstituted benzene substituents on adjacent carbons ortho-dichlorobenzene (1, 2 -dichlorobenzene) mp – 17. 2 o. C, bp 180. 4 o. C
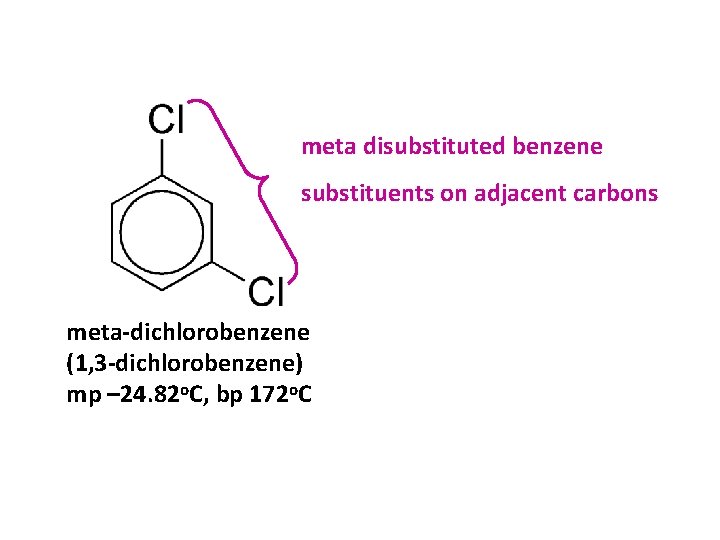
meta disubstituted benzene substituents on adjacent carbons meta-dichlorobenzene (1, 3 -dichlorobenzene) mp – 24. 82 o. C, bp 172 o. C

para disubstituted benzene substituents are on opposite sides of the benzene ring para-dichlorobenzene (1, 4 -dichlorobenzene) mp 53. 1, bp 174. 4 o. C
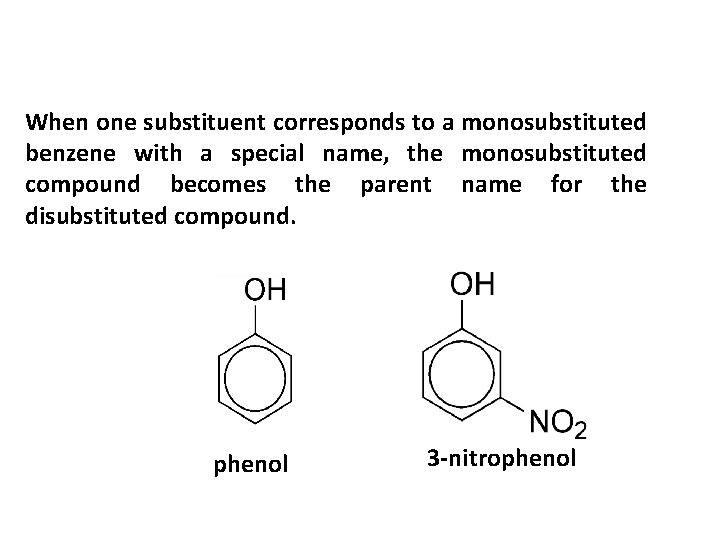
When one substituent corresponds to a monosubstituted benzene with a special name, the monosubstituted compound becomes the parent name for the disubstituted compound. phenol 3 -nitrophenol
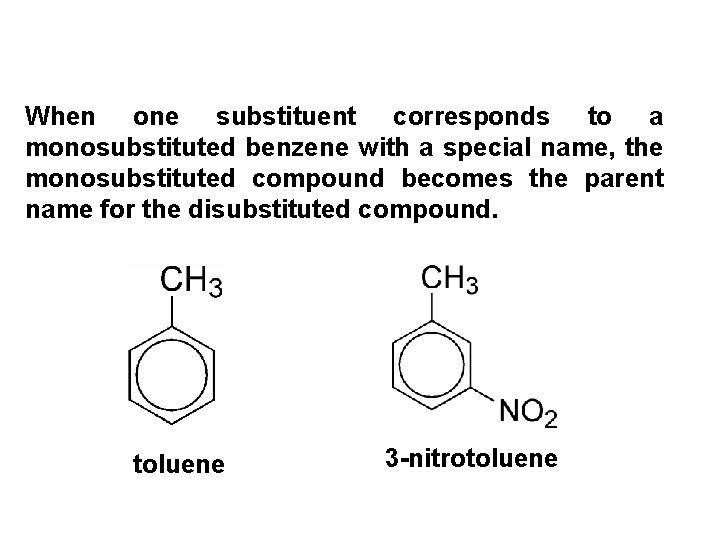
When one substituent corresponds to a monosubstituted benzene with a special name, the monosubstituted compound becomes the parent name for the disubstituted compound. toluene 3 -nitrotoluene
Tri- and Polysubstituted Benzenes

• When a benzene ring has three or more substituents, the carbon atoms in the ring are numbered. • Numbering starts at one of the substituent groups. • The numbering direction can be clockwise or counterclockwise. • Numbering must be in the direction that gives the substituent groups the lowest numbers.
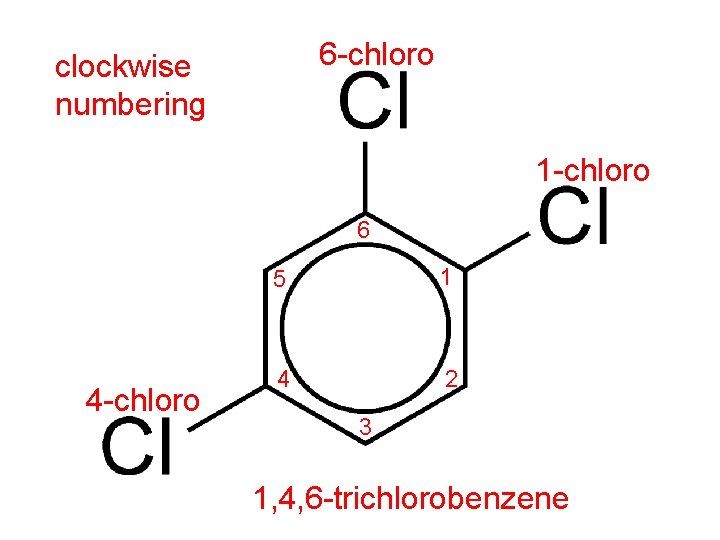
6 -chloro clockwise numbering 1 -chloro 6 4 -chloro 5 1 4 2 3 1, 4, 6 -trichlorobenzene

counterclockwise numbering chlorine substituents have lower numbers 4 -chloro 2 -chloro 1 -chloro 2 3 1 4 6 5 1, 2, 4 -trichlorobenzene

• When a compound is named as a derivative of the special parent compound, the substituent of the parent compound is considered to be C-1 of the ring.
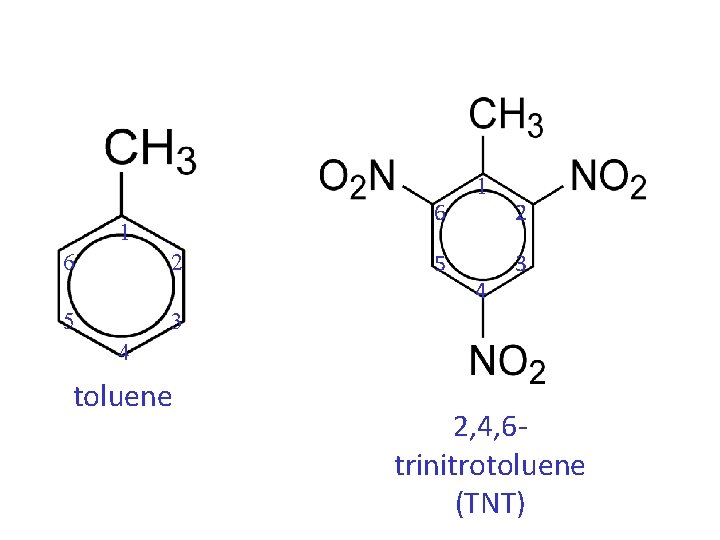
6 1 6 2 5 3 5 1 4 2 3 4 toluene 2, 4, 6 trinitrotoluene (TNT)
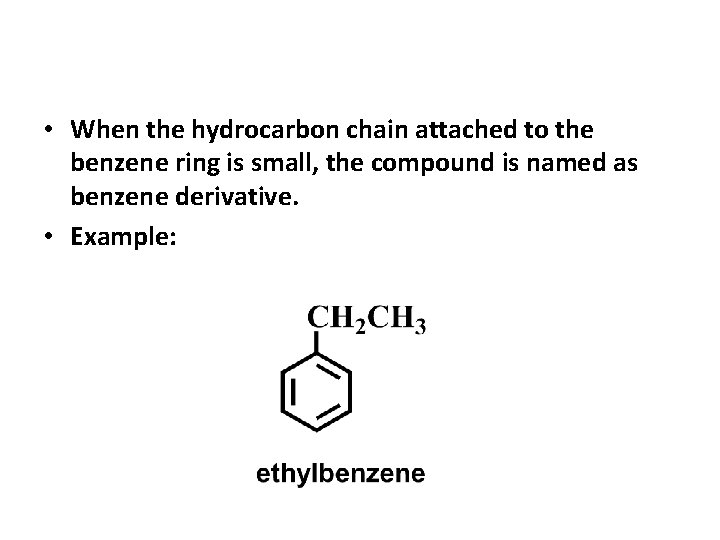
• When the hydrocarbon chain attached to the benzene ring is small, the compound is named as benzene derivative. • Example:









Benzene’s electrons participate as a Lewis base in reactions with Lewis acids Lewis acid: electron pair acceptor Lewis base: electron pair donor
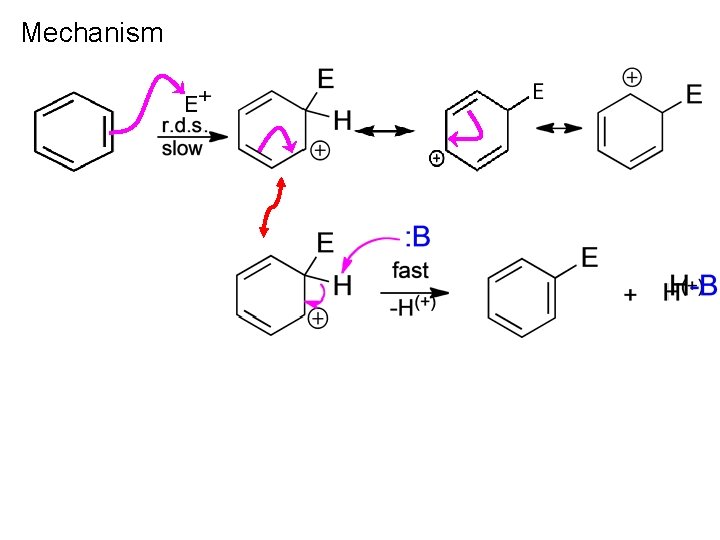
Mechanism
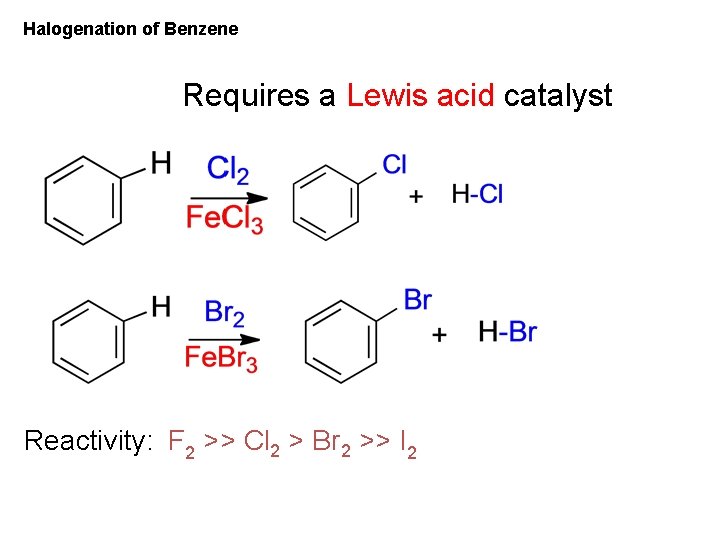
Halogenation of Benzene Requires a Lewis acid catalyst Reactivity: F 2 >> Cl 2 > Br 2 >> I 2
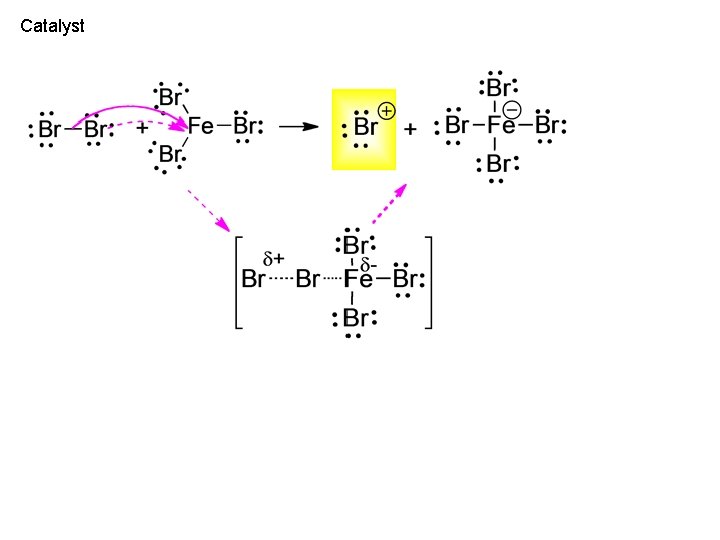
Catalyst
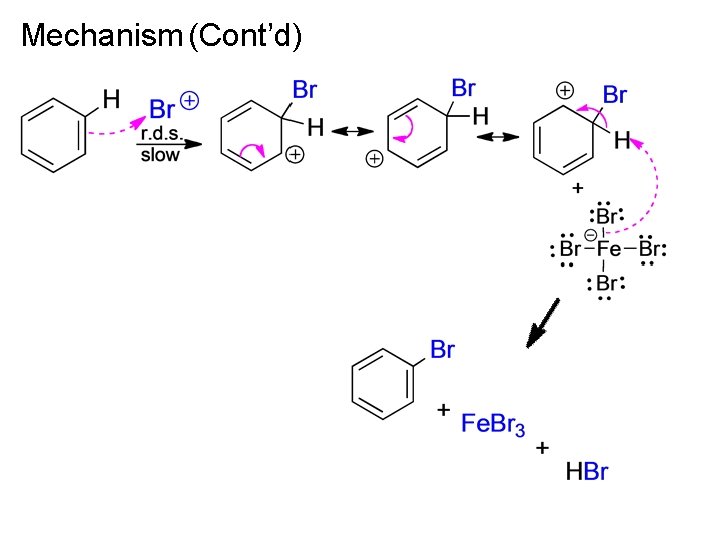
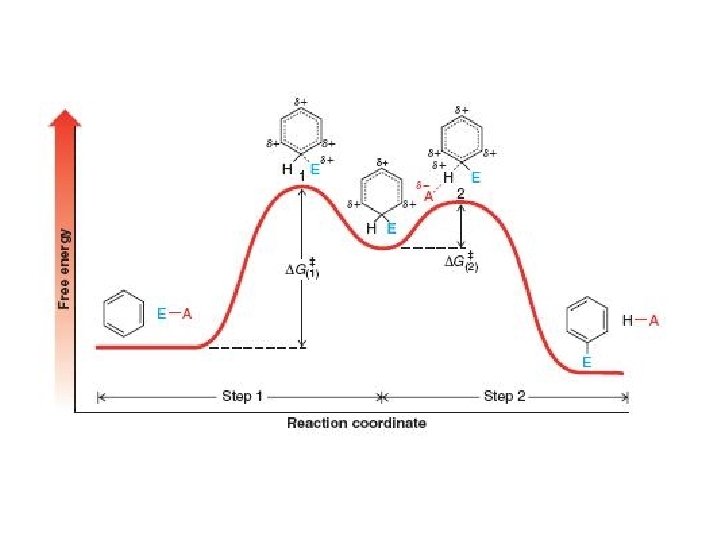
Mechanism (Cont’d)

Nitration of Benzene ⊕ Electrophile = NO 2 (nitronium ion)

Mechanism

Sulfonation r. d. s repeat next slide
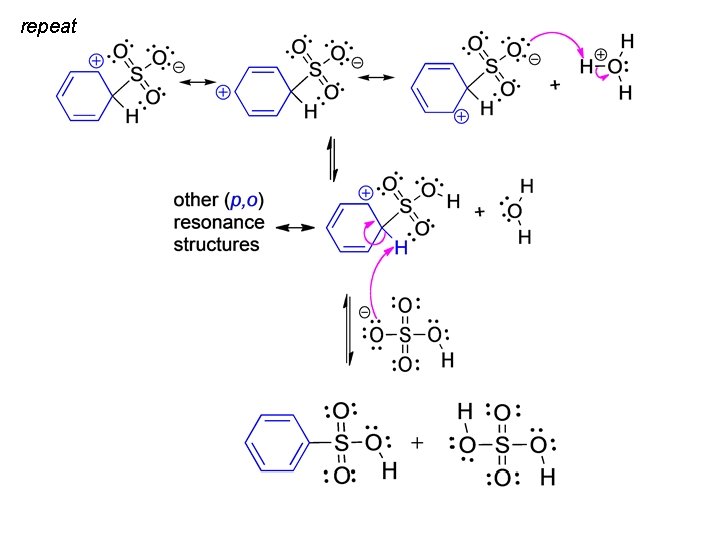
repeat
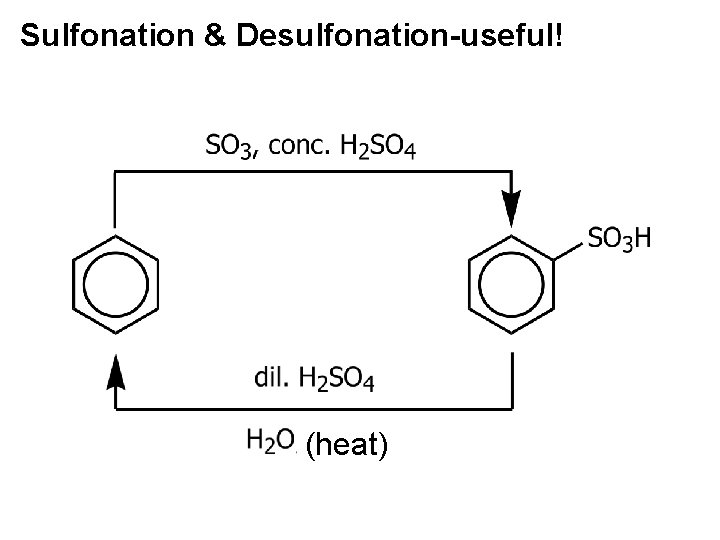
Sulfonation & Desulfonation-useful! (heat)
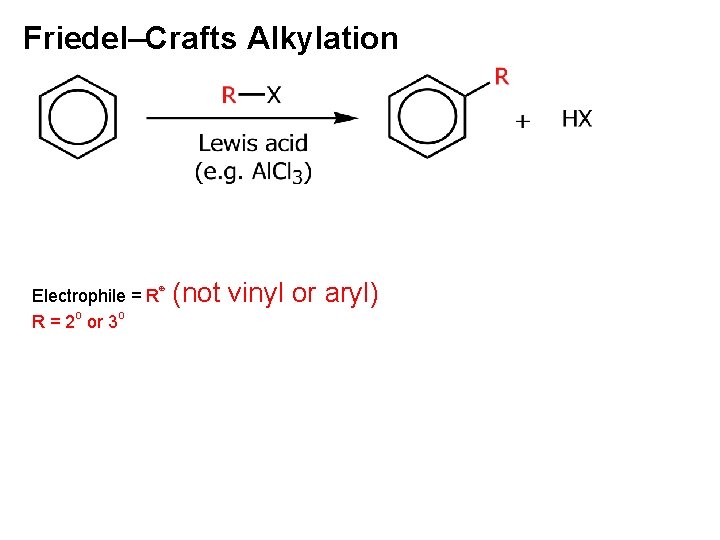
Friedel–Crafts Alkylation Electrophile = R⊕ R = 2 o or 3 o (not vinyl or aryl)
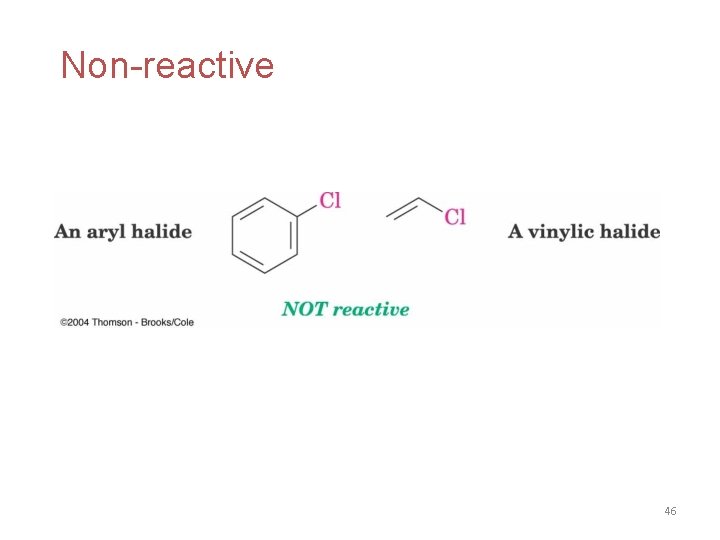
Non-reactive 46
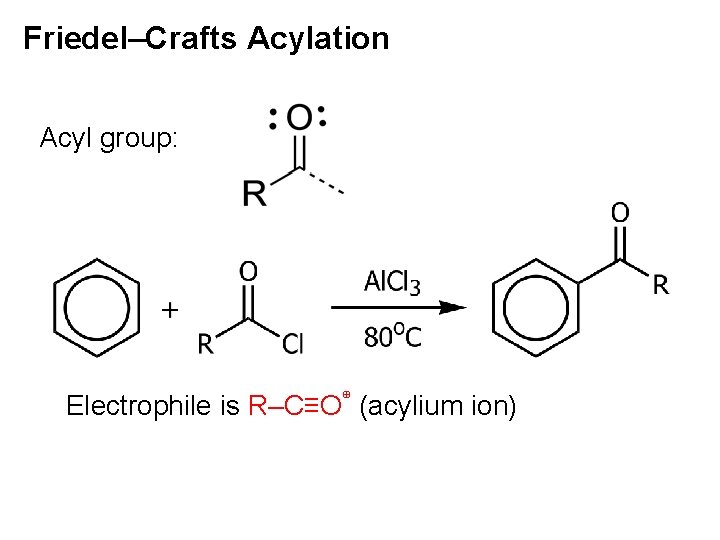
Friedel–Crafts Acylation Acyl group: Electrophile is R–C≡O⊕ (acylium ion)
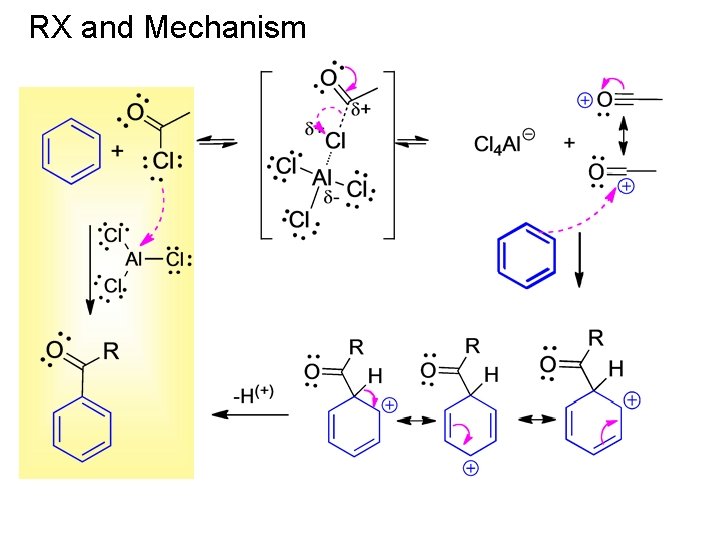
RX and Mechanism
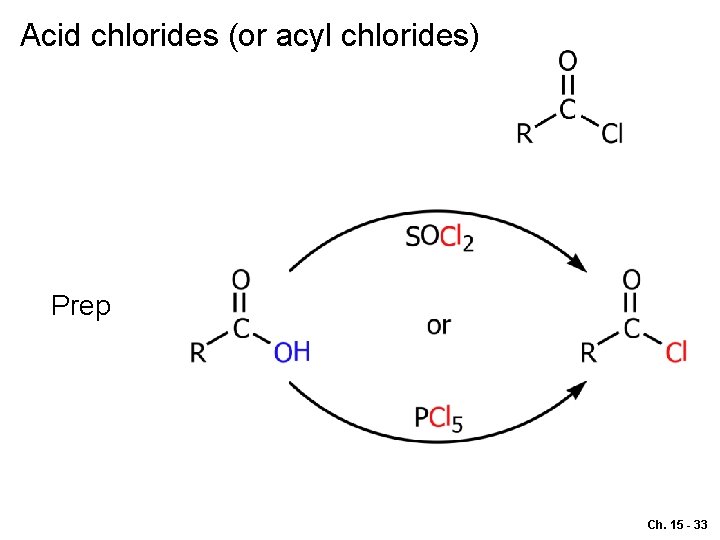
Acid chlorides (or acyl chlorides) Prep Ch. 15 - 33
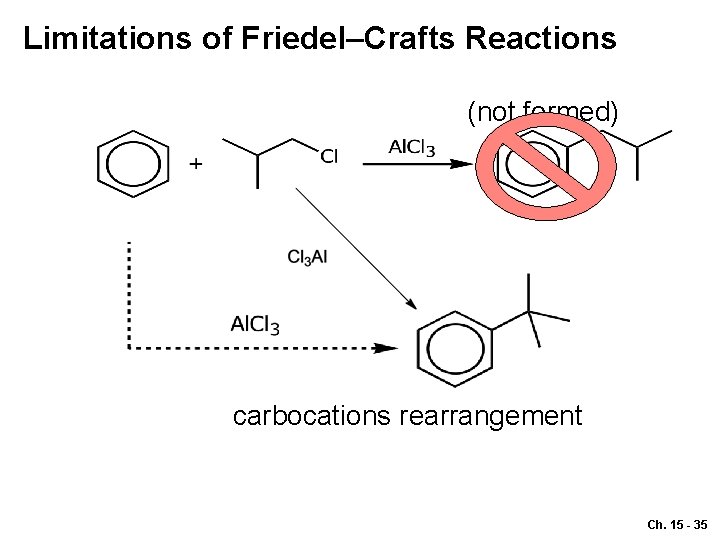
Limitations of Friedel–Crafts Reactions (not formed) carbocations rearrangement Ch. 15 - 35

Reason 1 o cation (not stable) 3 o cation Ch. 15 - 36
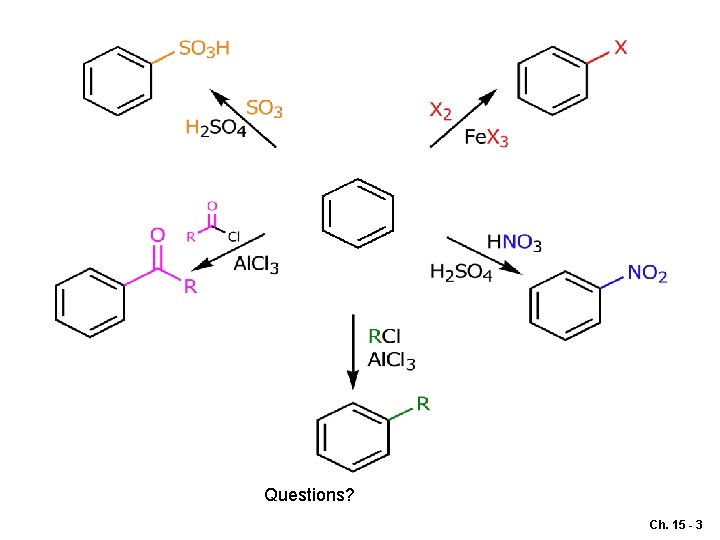
Questions? Ch. 15 - 3

Problems: Friedel–Crafts alkylations, acylations, etc. withdrawing groups & amines (basic) generally give poor yields deactivating gps

Basic amino groups (–NH 2, –NHR, & –NR 2) form strong electron withdrawing gps with acids Not Friedel-Crafts reactive
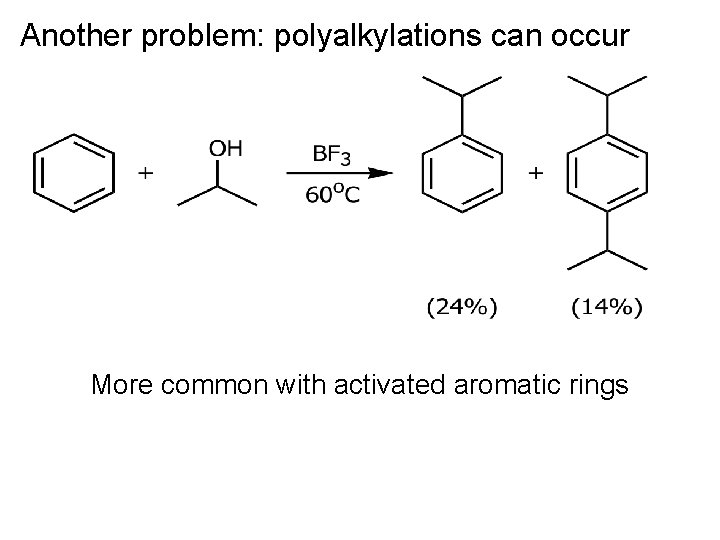
Another problem: polyalkylations can occur More common with activated aromatic rings

Electrophilic Aromatic Substitution Activating and Directing effects of substituents already on the ring

Substituents effect reactivity & regiochemistry of substitution possibilities ortho o meta m para p Y = EDG (electron-donating group) or EWG (electron-withdrawing group) Ch. 15 - 48

Products of Nitration 1 hr 48 hr 0. 0003 hr
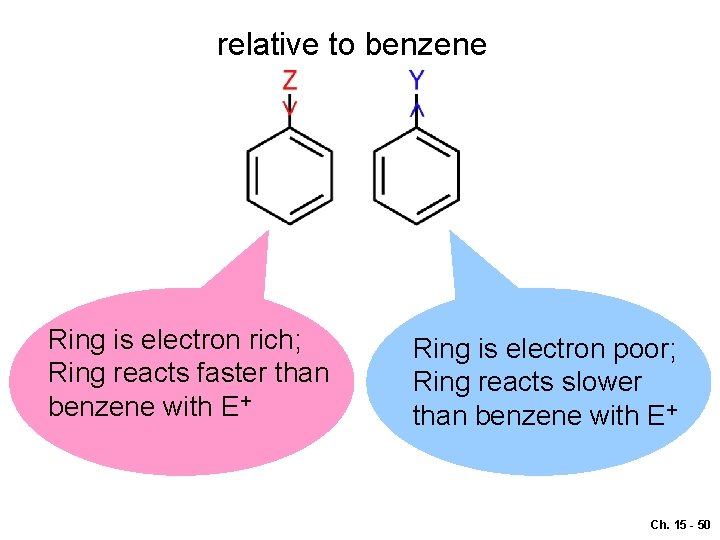
relative to benzene Ring is electron rich; Ring reacts faster than benzene with E+ Ring is electron poor; Ring reacts slower than benzene with E+ Ch. 15 - 50
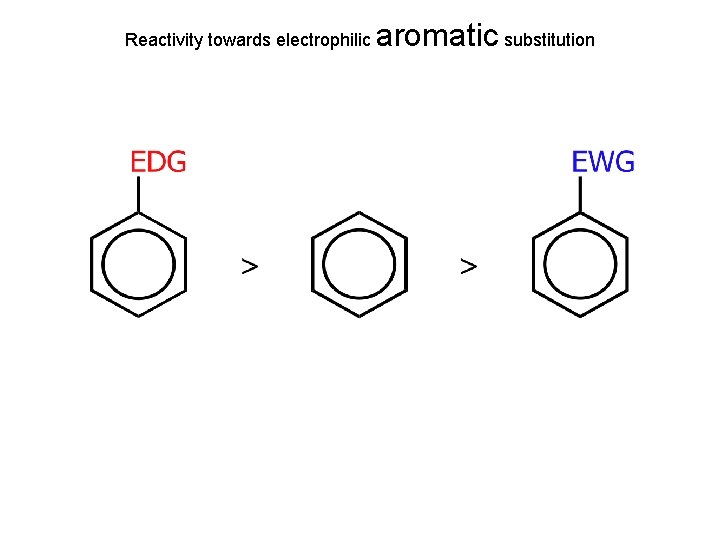
Reactivity towards electrophilic aromatic substitution

Regiochemistry: directing effect General aspects Either o-, p- directing or m-directing Rate-determining-step: aromatic ring -electrons attacking the E Ch. 15 - 57
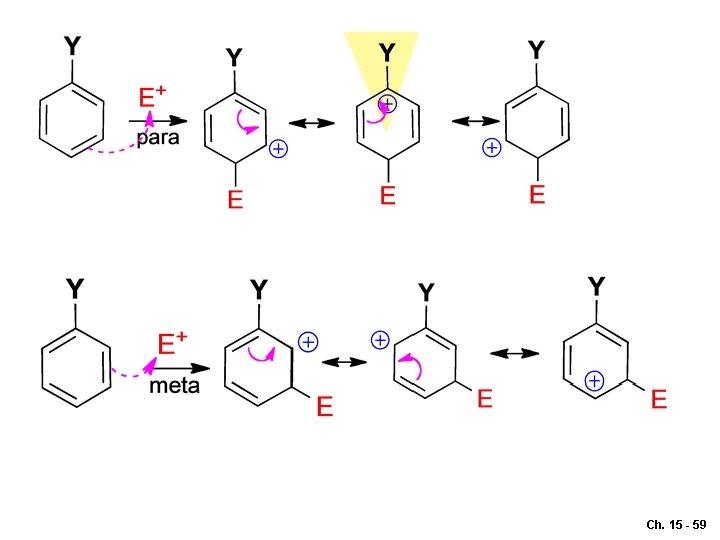
Ch. 15 - 59
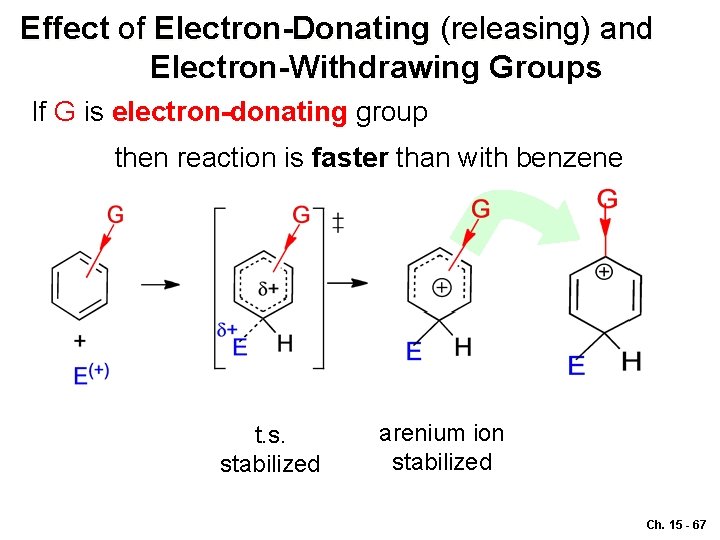
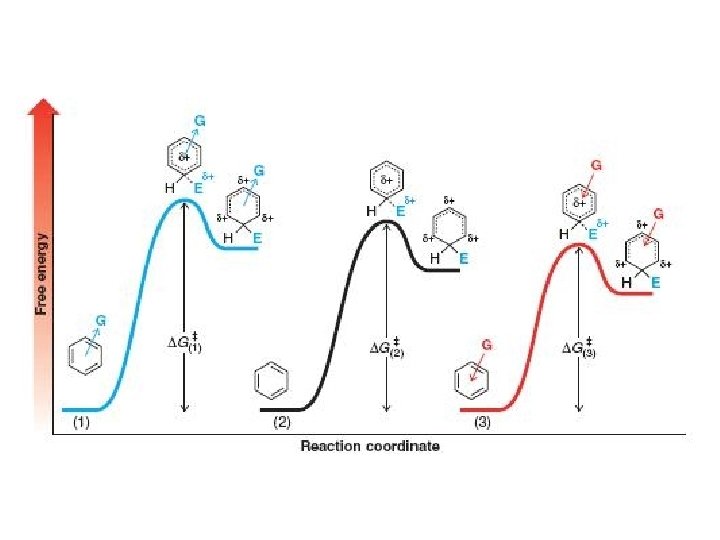
Effect of Electron-Donating (releasing) and Electron-Withdrawing Groups If G is electron-donating group then reaction is faster than with benzene t. s. stabilized arenium ion stabilized Ch. 15 - 67
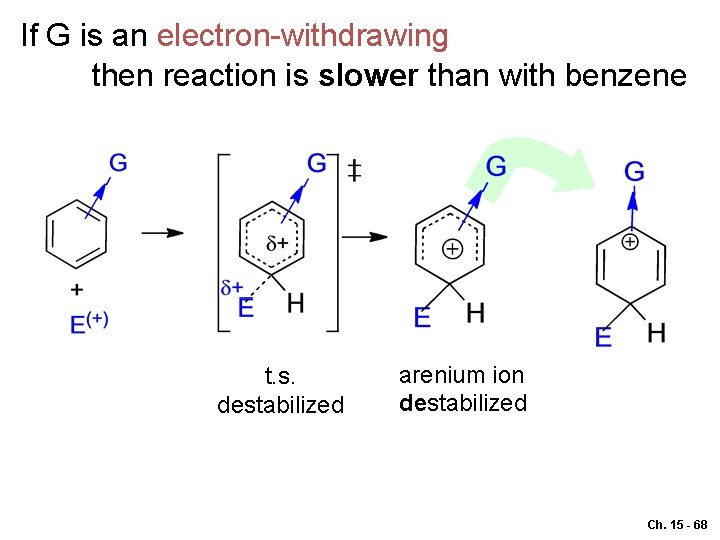
If G is an electron-withdrawing then reaction is slower than with benzene t. s. destabilized arenium ion destabilized Ch. 15 - 68
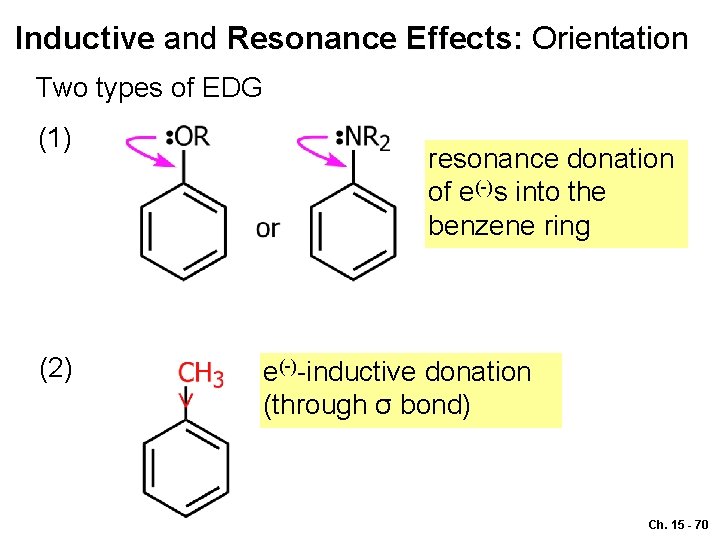
Inductive and Resonance Effects: Orientation Two types of EDG (1) (2) resonance donation of e(-)s into the benzene ring e(-)-inductive donation (through σ bond) Ch. 15 - 70
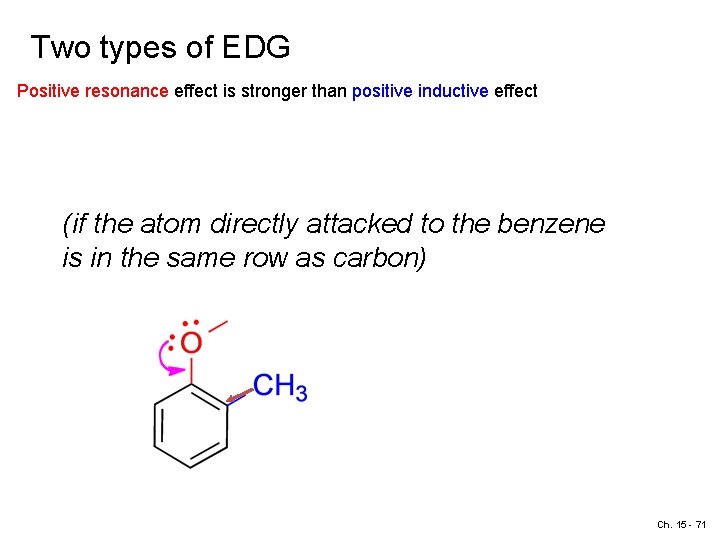
Two types of EDG Positive resonance effect is stronger than positive inductive effect (if the atom directly attacked to the benzene is in the same row as carbon) Ch. 15 - 71
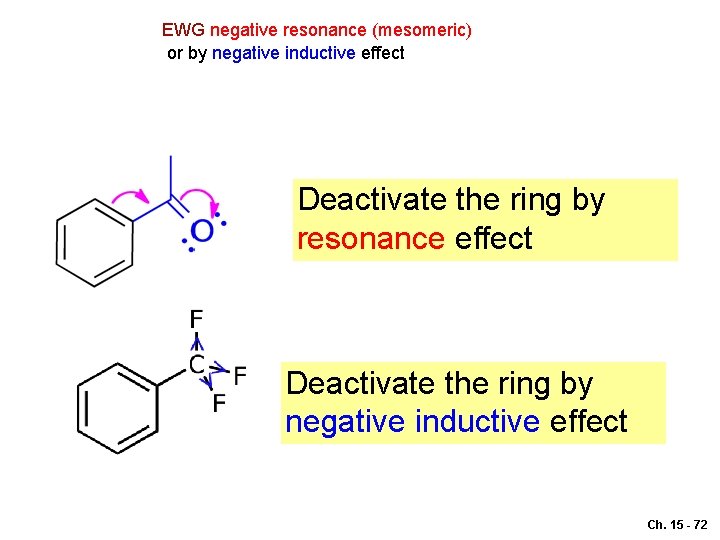
EWG negative resonance (mesomeric) or by negative inductive effect Deactivate the ring by resonance effect Deactivate the ring by negative inductive effect Ch. 15 - 72
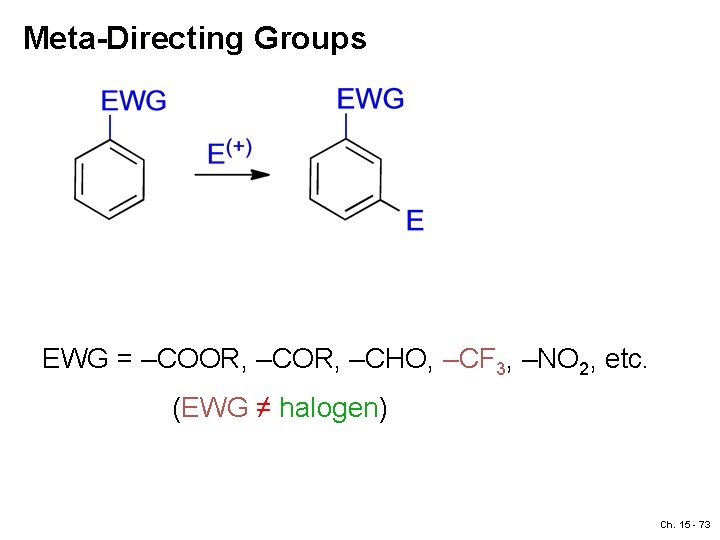
Meta-Directing Groups EWG = –COOR, –CHO, –CF 3, –NO 2, etc. (EWG ≠ halogen) Ch. 15 - 73
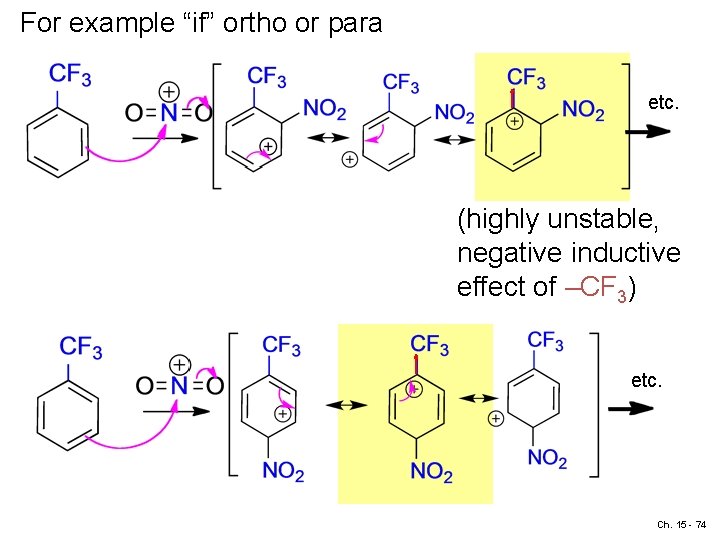
For example “if” ortho or para etc. (highly unstable, negative inductive effect of –CF 3) etc. Ch. 15 - 74
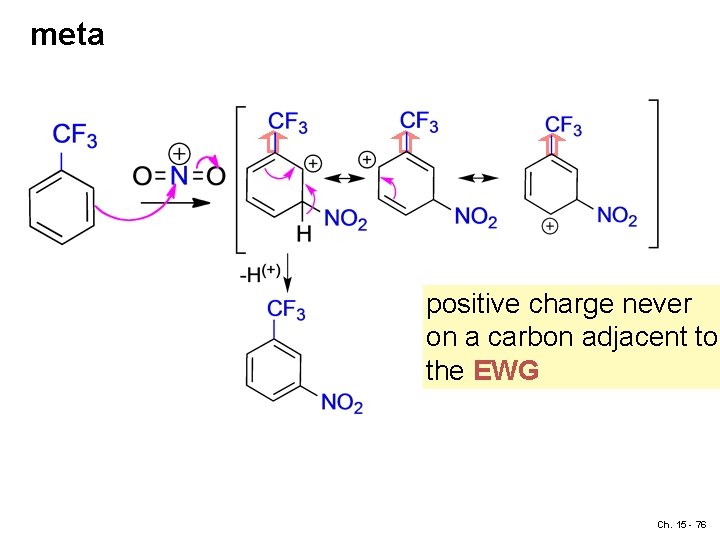
meta positive charge never on a carbon adjacent to the EWG Ch. 15 - 76

Ortho–Para-Directing Groups EDG = –NR 2, –OR, –OH, etc. Ch. 15 - 77
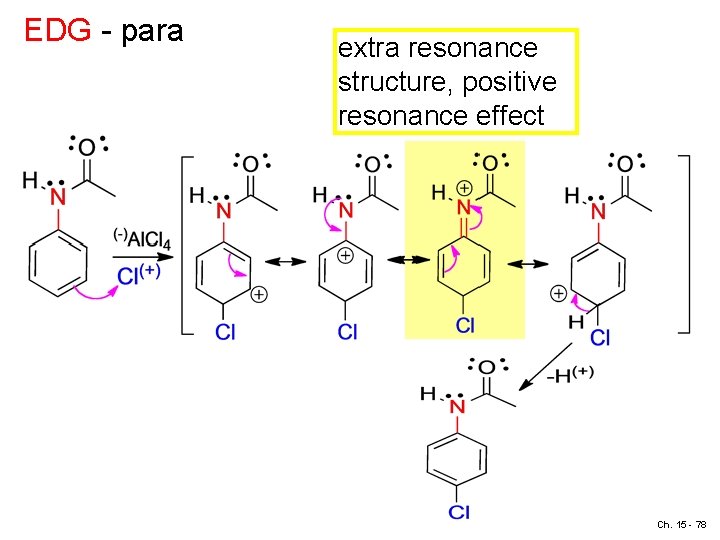
EDG - para extra resonance structure, positive resonance effect Ch. 15 - 78
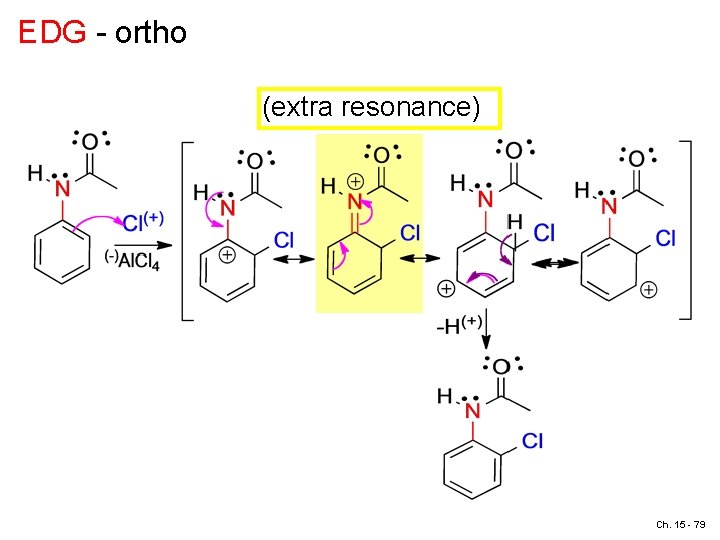
EDG - ortho (extra resonance) Ch. 15 - 79
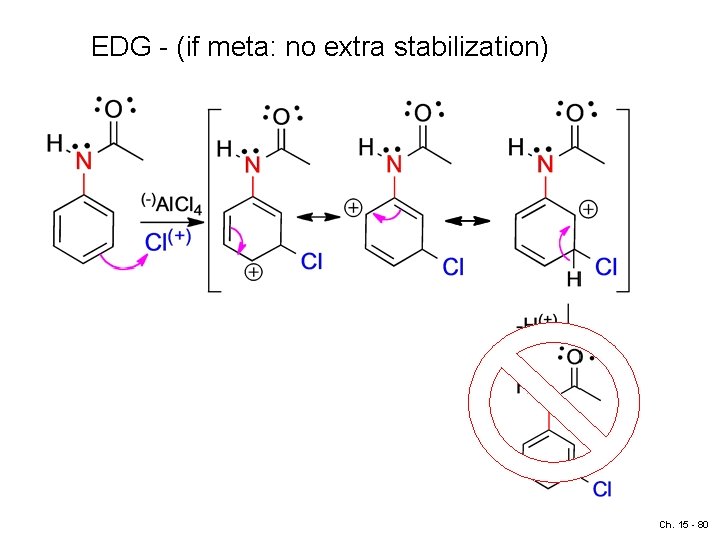
EDG - (if meta: no extra stabilization) Ch. 15 - 80
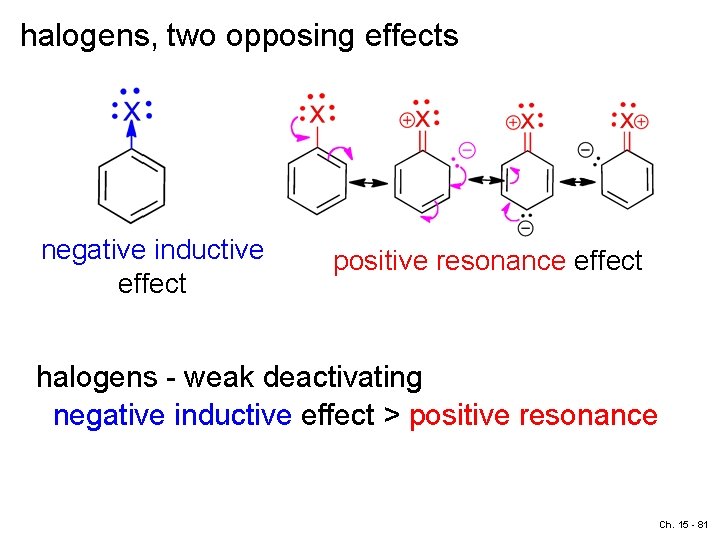
halogens, two opposing effects negative inductive effect positive resonance effect halogens - weak deactivating negative inductive effect > positive resonance Ch. 15 - 81
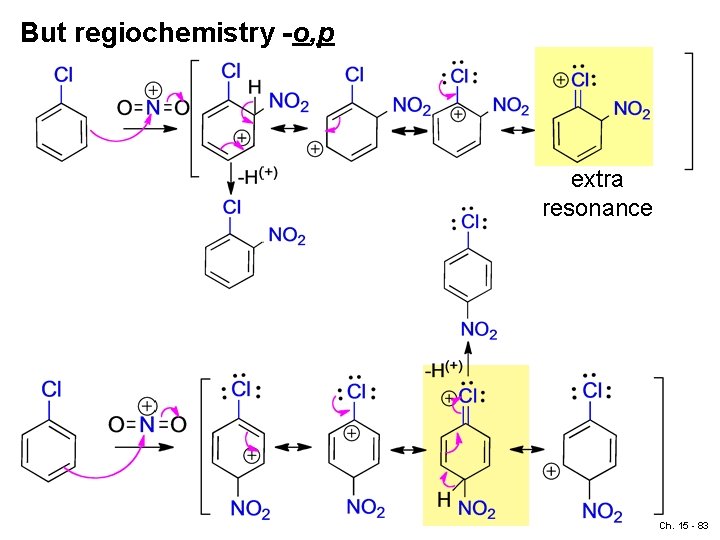
But regiochemistry -o, p extra resonance Ch. 15 - 83
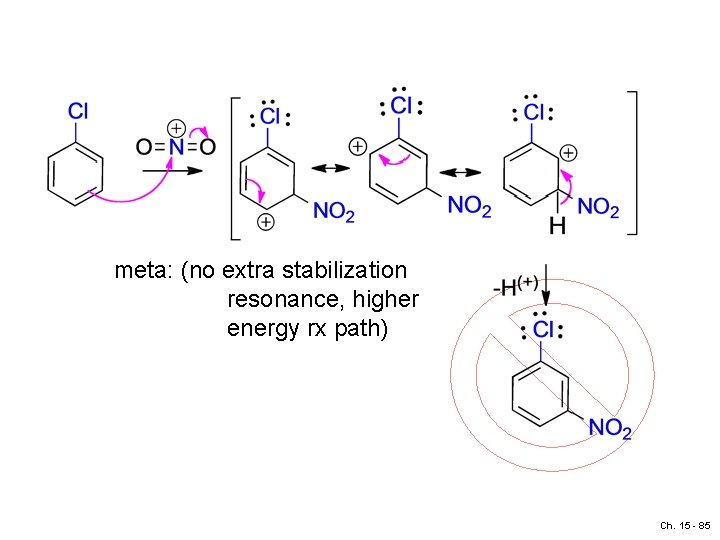
meta: (no extra stabilization resonance, higher energy rx path) Ch. 15 - 85
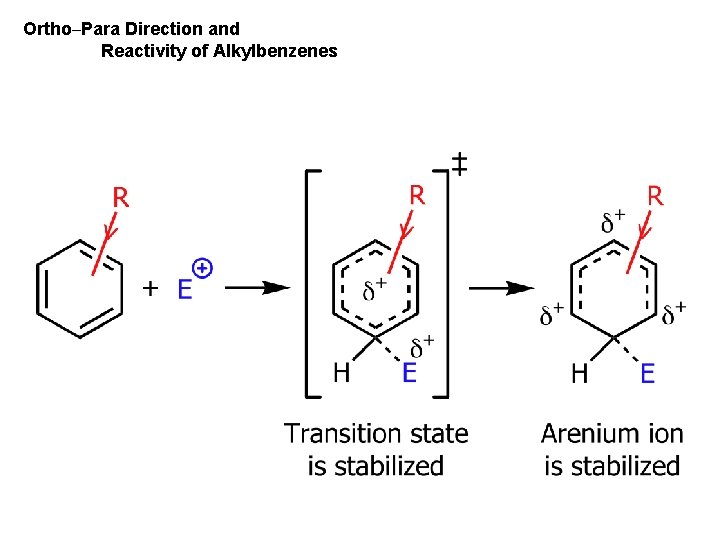
Ortho–Para Direction and Reactivity of Alkylbenzenes
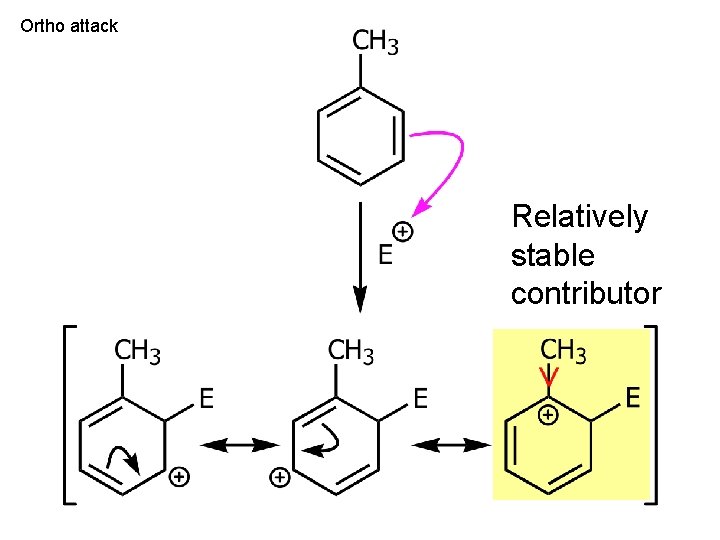
Ortho attack Relatively stable contributor
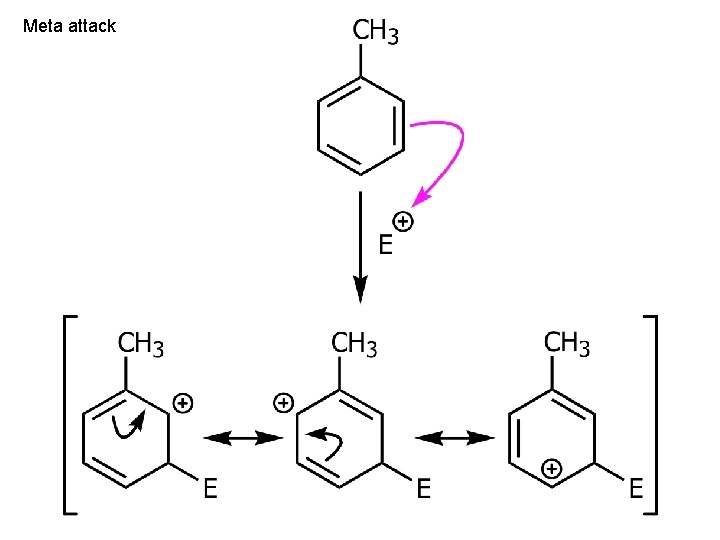
Meta attack
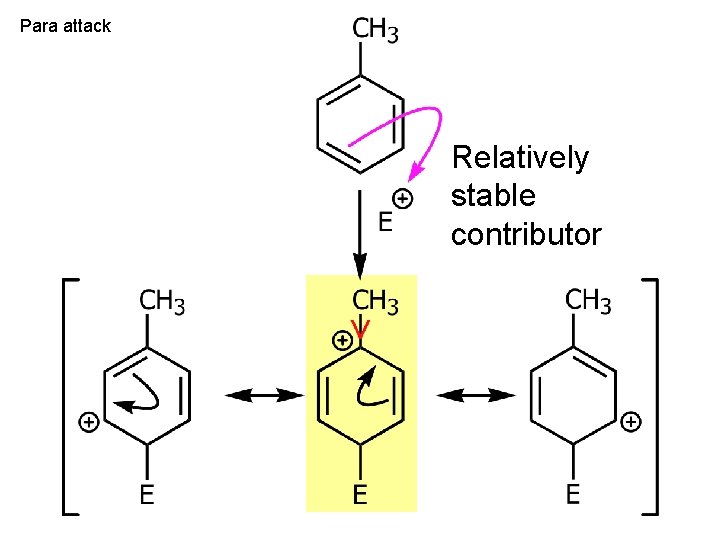
Para attack Relatively stable contributor


Classification of Substituents
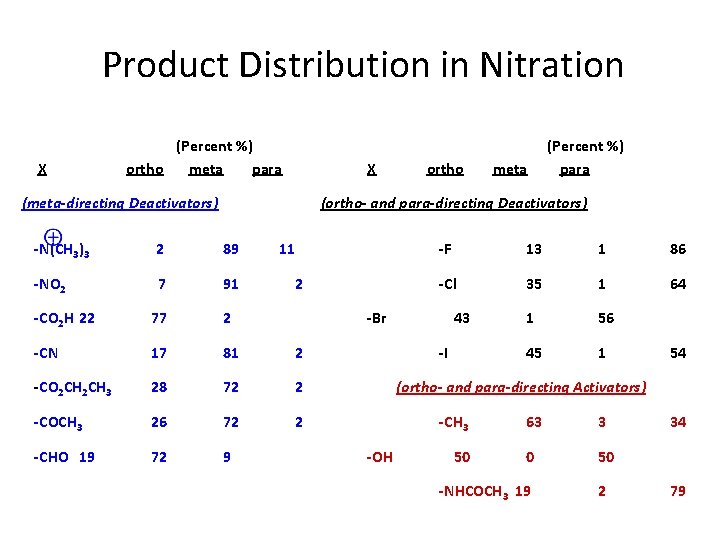
Product Distribution in Nitration X (Percent %) ortho meta para X (meta-directing Deactivators) ortho meta (Percent %) para (ortho- and para-directing Deactivators) -N(CH 3)3 2 89 11 -NO 2 7 91 -CO 2 H 22 77 2 -CN 17 81 2 -CO 2 CH 3 28 72 2 -COCH 3 26 72 2 -CHO 19 72 9 2 -F 13 1 86 -Cl 35 1 64 1 56 45 1 -Br 43 -I 54 (ortho- and para-directing Activators) -CH 3 -OH 50 63 3 0 50 -NHCOCH 3 19 2 34 79

Summary
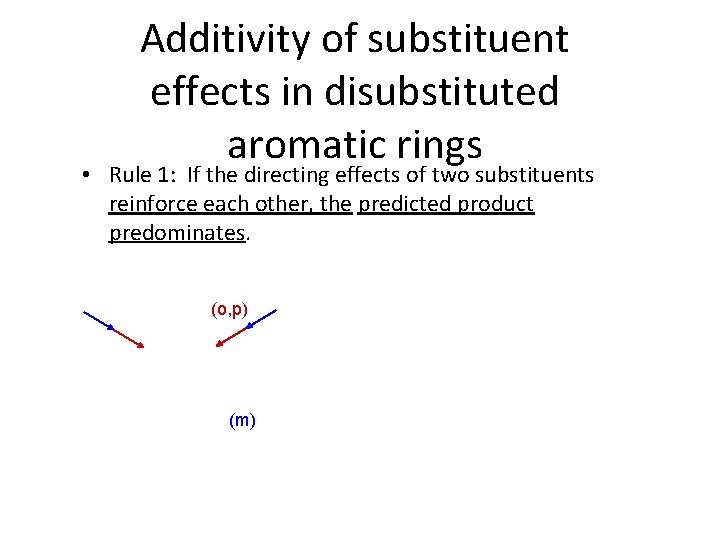
Additivity of substituent effects in disubstituted aromatic rings • Rule 1: If the directing effects of two substituents reinforce each other, the predicted product predominates. (o, p) (m)
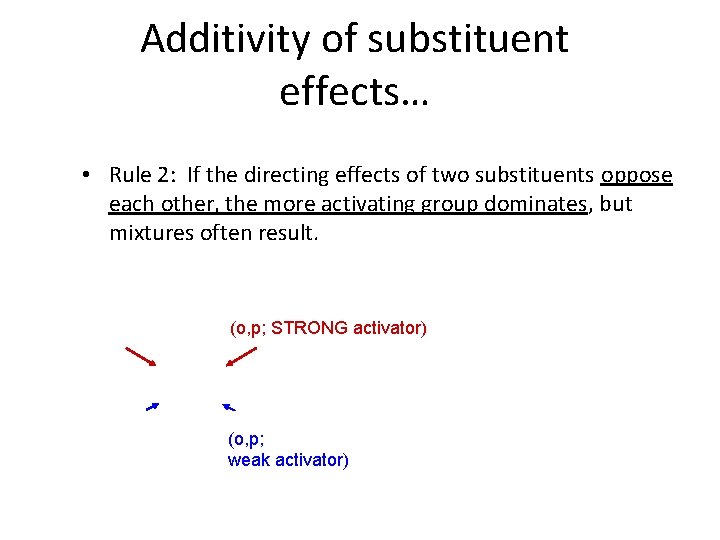
Additivity of substituent effects… • Rule 2: If the directing effects of two substituents oppose each other, the more activating group dominates, but mixtures often result. (o, p; STRONG activator) (o, p; weak activator)
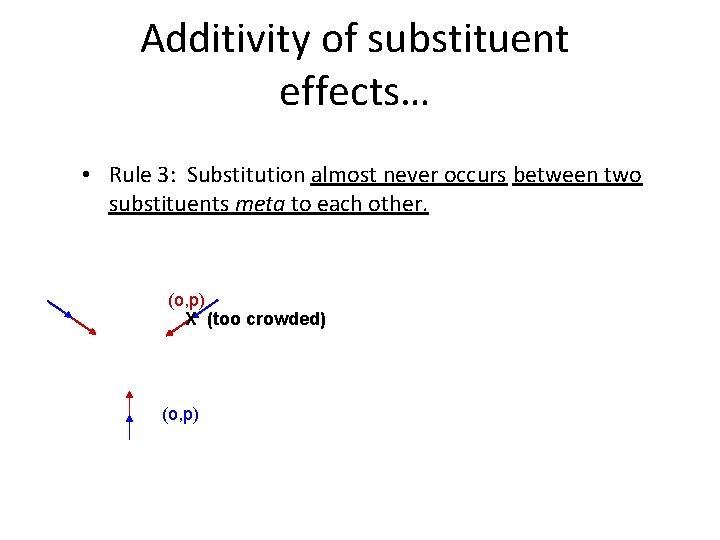
Additivity of substituent effects… • Rule 3: Substitution almost never occurs between two substituents meta to each other. (o, p) X (too crowded) (o, p)

Additivity of substituent effects… • Rule 4: With a bulky o, p- director and/or a bulky electrophile, para substitution predominates. (o, p; BULKY) (HSO 3+ is a BULKY electrophile)

- Slides: 91