Aqueous Solutions Water is the dissolving medium or











- Slides: 26

Aqueous Solutions Water is the dissolving medium, or solvent.

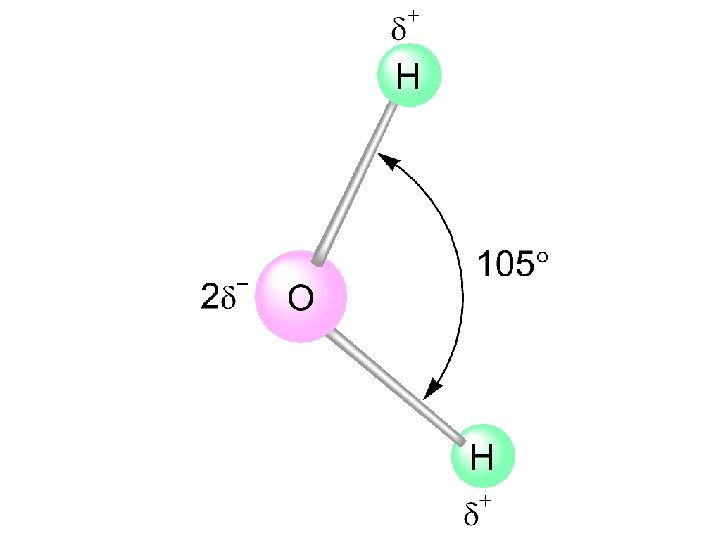
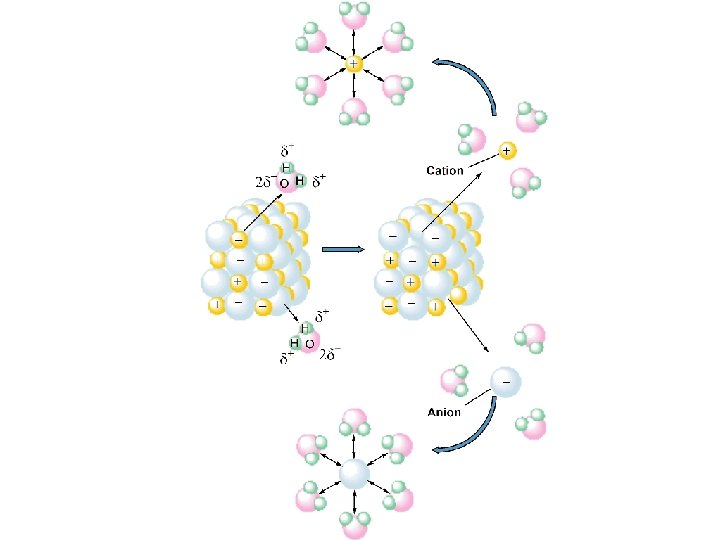
Some Properties of Water - Water is “bent” or V-shaped. The O-H bonds are covalent. Water is a polar molecule. Hydration occurs when salts dissolve in water.



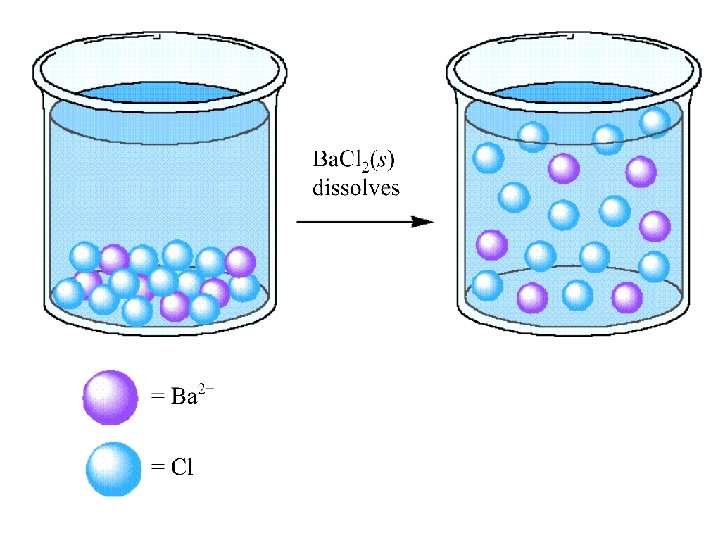
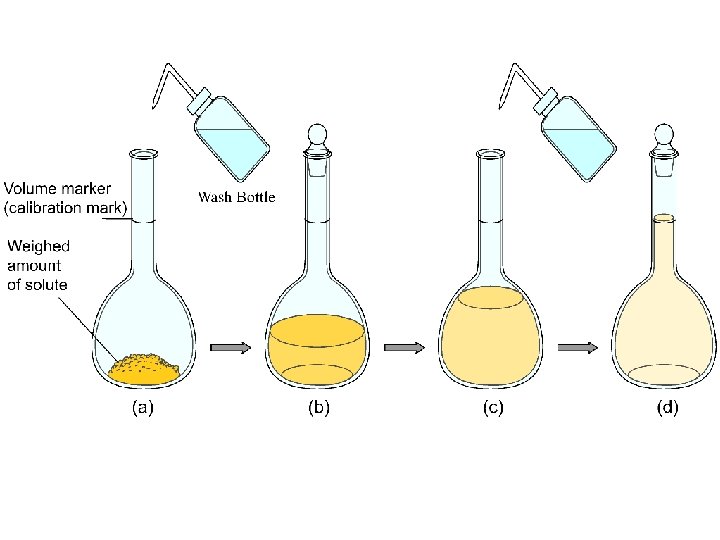
A Solute - dissolves in water (or other “solvent”) - changes phase (if different from the solvent) - is present in lesser amount (if the same phase as the solvent)


A Solvent - retains its phase (if different from the solute) - is present in greater amount (if the same phase as the solute)

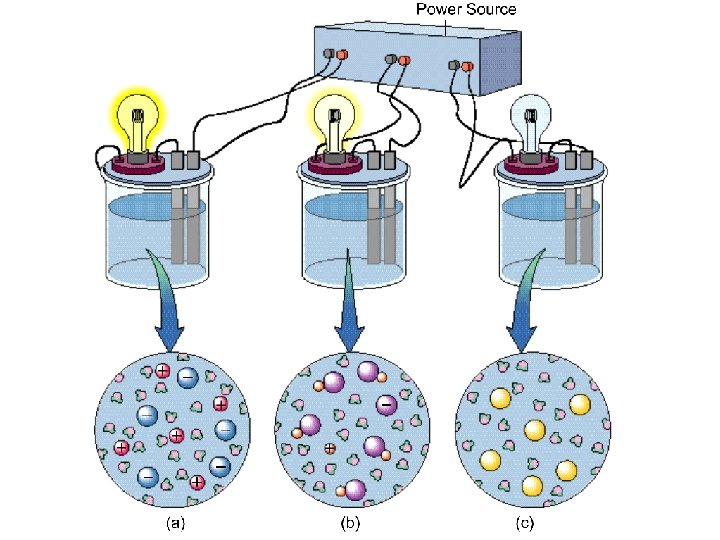
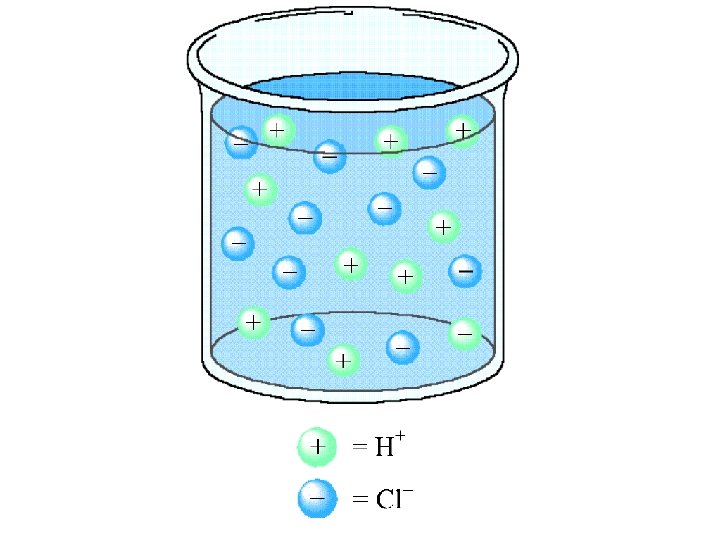
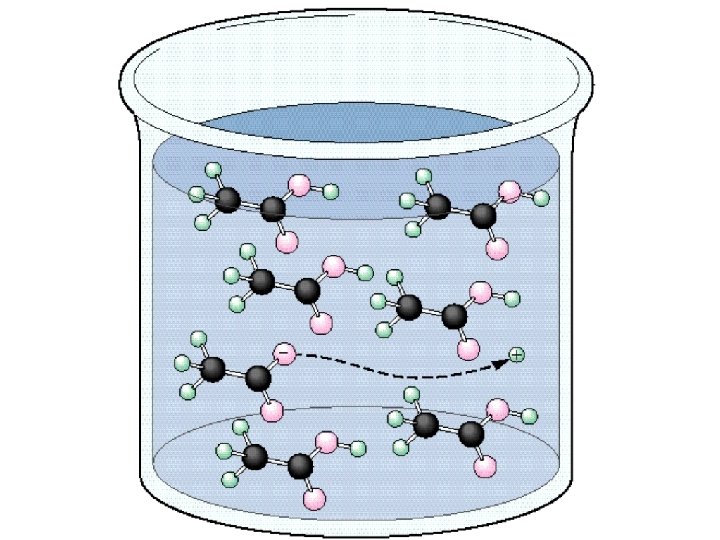
Electrolytes Strong - conduct current efficiently Na. Cl, HNO 3 Weak - conduct only a small current vinegar, tap water Non - no current flows pure water, sugar solution


Acids Strong acids - dissociate completely to produce H+ in solution hydrochloric and sulfuric acid Weak acids - dissociate to a slight extent to give H+ in solution acetic and formic acid




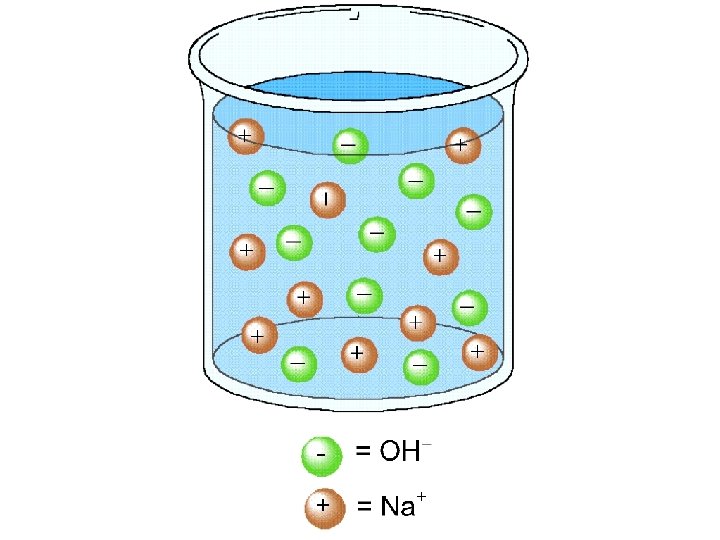
Bases Strong bases - react completely with water to give OH ions. sodium hydroxide Weak bases - react only slightly with water to give OH ions. ammonia

Strong Bases


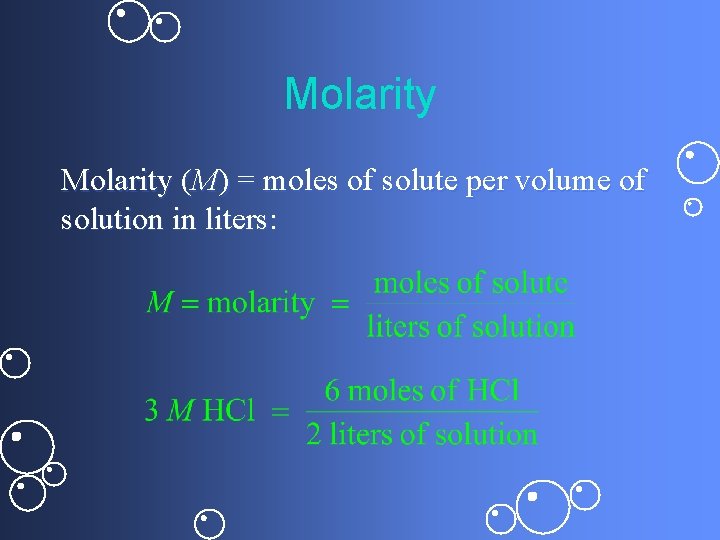
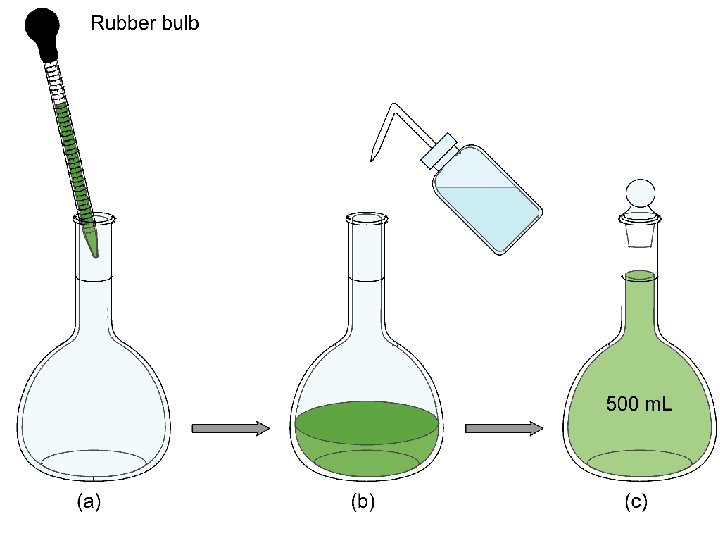
Molarity (M) = moles of solute per volume of solution in liters:


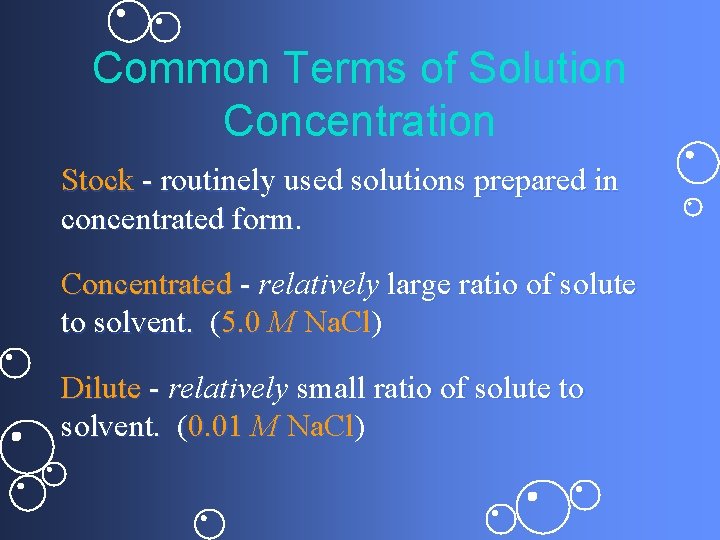
Common Terms of Solution Concentration Stock - routinely used solutions prepared in concentrated form. Concentrated - relatively large ratio of solute to solvent. (5. 0 M Na. Cl) Dilute - relatively small ratio of solute to solvent. (0. 01 M Na. Cl)


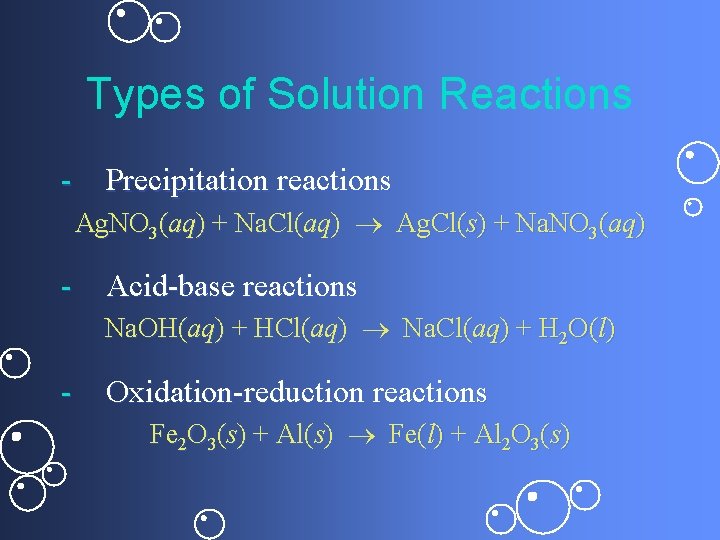
Types of Solution Reactions - Precipitation reactions Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(s) + Na. NO 3(aq) - Acid-base reactions Na. OH(aq) + HCl(aq) Na. Cl(aq) + H 2 O(l) - Oxidation-reduction reactions Fe 2 O 3(s) + Al(s) Fe(l) + Al 2 O 3(s)

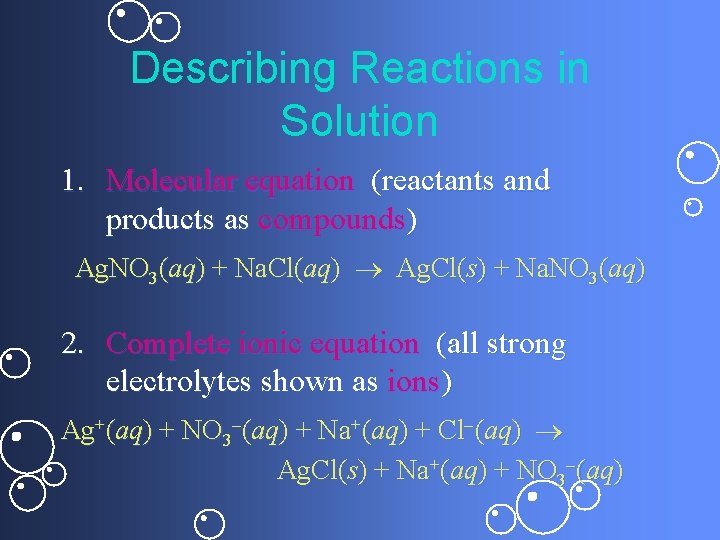
Describing Reactions in Solution 1. Molecular equation (reactants and products as compounds) Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(s) + Na. NO 3(aq) 2. Complete ionic equation (all strong electrolytes shown as ions) Ag+(aq) + NO 3 (aq) + Na+(aq) + Cl (aq) Ag. Cl(s) + Na+(aq) + NO 3 (aq)

Describing Reactions in Solution (continued) 3. Net ionic equation (show only components that actually react) Ag+(aq) + Cl (aq) Ag. Cl(s) Na+ and NO 3 are spectator ions.

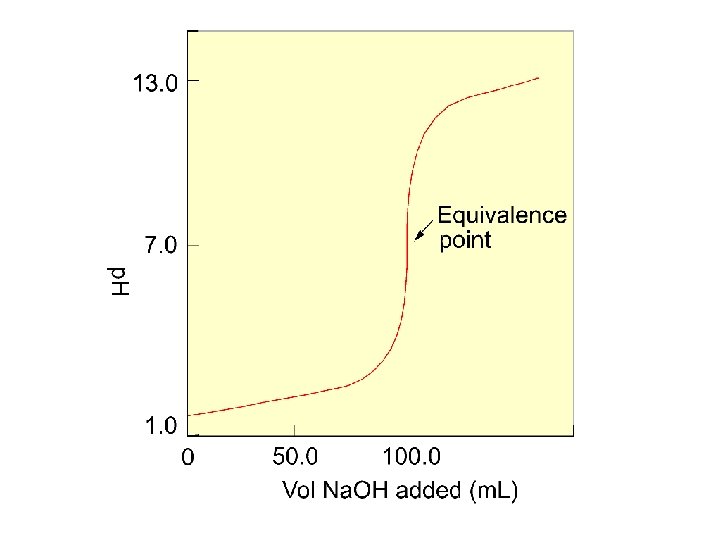
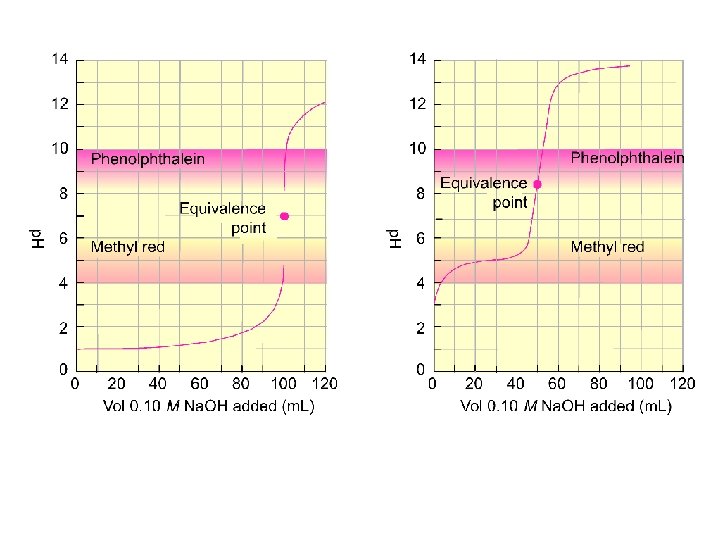
Key Titration Terms Titrant - solution of known concentration used in titration Analyte - substance being analyzed Equivalence point - enough titrant added to react exactly with the analyte Endpoint - the indicator changes color so you can tell the equivalence point has been reached.

