AQA GCSE Design and Technology 8552 3 Metals























- Slides: 27

AQA GCSE Design and Technology 8552 3 Metals and alloys Unit 3 Materials and their working properties

Objectives • Know the primary sources of materials for producing metals and alloys • Be able to recognise and characterise different types of metals and alloys • Understand how the physical and working properties of a range of metals and alloys affect their performance

Metals and alloys Unit 3 Materials and their working properties Metal • Metals have been essential in the development of civilisation • The word ‘metal’ comes from the ancient Greek word ‘metallon’ which means to mine, excavate or extract from the ground • Prehistoric man used metals to make tools and weapons • Which common metals would you find in a domestic kitchen?

Metals and alloys Unit 3 Materials and their working properties Ore • The Earth’s crust contains many types of rock • Metallic minerals are found naturally in rock or ore • Ore is obtained by mining and the metals within it are extracted • The method used for extraction depends on the metal’s reactivity with air, water or acids • The more reactive the metal, the more expensive it is to extract

Metals and alloys Unit 3 Materials and their working properties Finding metal • Some naturally occurring metals are found as lumps in the rock and don’t require a chemical extraction • Why is gold expensive in comparison to aluminium?

Metals and alloys Unit 3 Materials and their working properties Extraction processes • Some metals exist as oxides • Metals such as copper, iron and zinc are oxides. These are heated with carbon to extract the metal • Electrolysis is used to extract metals such as aluminium • Are metals a renewable resource? • How can man reduce the speed at which we will run out of natural resources?

Metals and alloys Unit 3 Materials and their working properties Iron ore • Iron can be extracted from iron ore by using a blast furnace and the process of smelting • Smelting extracts common industrial metals such as iron • The extreme heat draws off the metals in a liquid state called ‘hot metal’ • The impurities are removed and the iron mixed or ‘alloyed’ with a small amount of carbon to create steel • The amount of added carbon affects the strength of the steel

Metals and alloys Unit 3 Materials and their working properties Ferrous metals • Ferrous metals contain iron and may rust • Iron and steel can corrode – this is known as rust • Rust is a compound called iron oxide and is formed when iron and oxygen react in the presence of moisture or water • Most ferrous metals are magnetic • Name two types of protective layer that could be added to metal to help prevent rust

Metals and alloys Unit 3 Materials and their working properties Ductility and malleability • Ductile metals • Will stretch without being damaged • Can be drawn or stretched out into long wires • Copper is highly ductile and can be drawn into long, thin wires • Malleable metals • Can be hammered into a shape without breaking • Can be rolled or pressed into sheets • Will deform under compression

Metals and alloys Unit 3 Materials and their working properties Hard or tough? • Hardness – a material’s ability to withstand abrasion • A very hard metal is likely to crack or shatter upon impact or force. Cast iron would be described as hard and brittle • Toughness – metal requires strength and ductility • This is how well a metal can absorb energy and resist fracturing without deforming

Metals and alloys Unit 3 Materials and their working properties Tensile strength • Tensile strength is the amount of tensile stress (stretching) a material can withstand before breaking or failing • Testing for tensile strength is crucial in industry • Suggest two uses for metals with high tensile strength • Why is destructive testing so important?

Metals and alloys Unit 3 Materials and their working properties Hard as nails • Steel is a ferrous metal available in different forms • Steel has a high resistance to corrosion, staining and friction which makes it suitable for a wide range of uses • These elements give steel its characteristic properties of hardness and toughness • High speed steel is formed by alloying elements of carbon, tungsten, vanadium, cobalt, chromium or molybdenum • Name three everyday applications of stainless steel

Metals and alloys Unit 3 Materials and their working properties Material selection • Ferrous metals have varying properties making them suitable for different uses • Low carbon steel is tough, ductile and easily welded • High carbon steel is very hard wearing, but less ductile or malleable • Cast iron is hard and easily cast into shapes, but brittle • Suggest two items made from each of these metals?

Metals and alloys Unit 3 Materials and their working properties Non-ferrous properties • Non-ferrous metals don’t contain iron • They are often more expensive than ferrous metals owing to their desirable properties which include: • Lightweight • Good conductivity • Ductile and malleable • Resistant to corrosion • The process of galvanising adds a protective coat of zinc to iron and steel to help prevent rusting

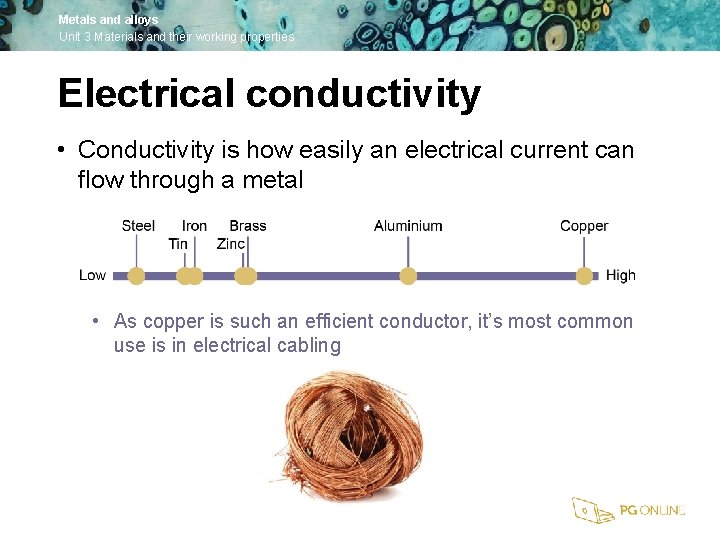
Metals and alloys Unit 3 Materials and their working properties Electrical conductivity • Conductivity is how easily an electrical current can flow through a metal • As copper is such an efficient conductor, it’s most common use is in electrical cabling

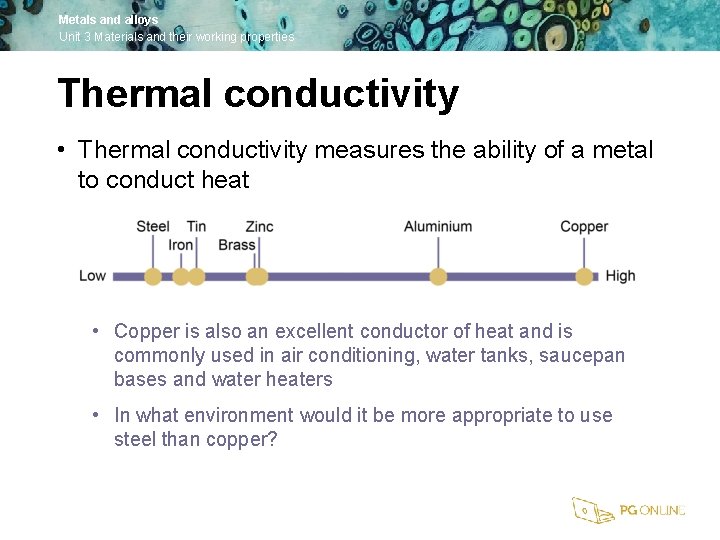
Metals and alloys Unit 3 Materials and their working properties Thermal conductivity • Thermal conductivity measures the ability of a metal to conduct heat • Copper is also an excellent conductor of heat and is commonly used in air conditioning, water tanks, saucepan bases and water heaters • In what environment would it be more appropriate to use steel than copper?

Metals and alloys Unit 3 Materials and their working properties Non-ferrous metals • Aluminium, copper, tin and zinc are all non-ferrous metals • List a selection of household items that may be made from non-ferrous metals • In what way do non-ferrous metals differ from ferrous metals?

Metals and alloys Unit 3 Materials and their working properties Tin • Tin is commonly associated with a ‘tin can’ • In fact, the can is steel, with a thin coating of tin, ‘tin plating’, which helps preserve the contents • Tin is soft, ductile and malleable • Alloys of tin include pewter, copper, bronze and soft solder • What other non-ferrous metal is associated with the production of cans?

Metals and alloys Unit 3 Materials and their working properties Alloys • Metals are rarely used in their pure form. Alloys are made by combining two or more elements • This helps improve the working properties and appearance • Brass and steel are common alloys • Stainless steel is made by combining iron, with a small amount of carbon and chromium • This protects the alloy from oxygen – what will this help prevent? • What are the advantages of creating alloys?

Metals and alloys Unit 3 Materials and their working properties Worksheet • Complete Tasks 1 and 2 on your worksheet

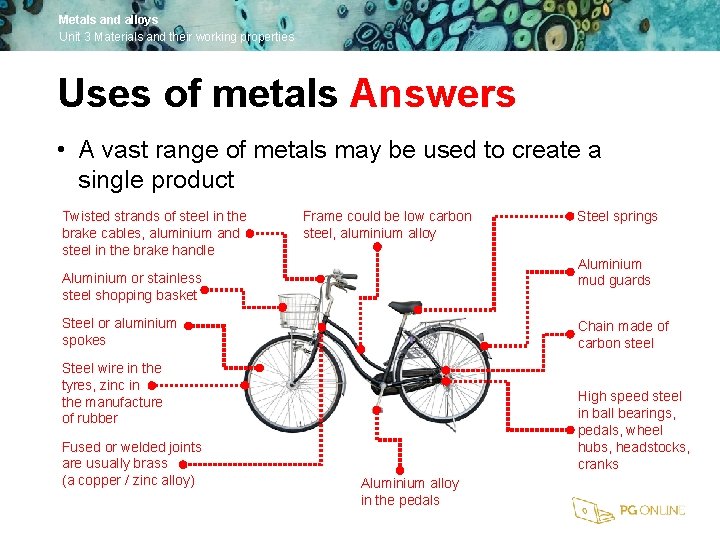
Metals and alloys Unit 3 Materials and their working properties Uses of metals • Given the various properties of metals, list as many metals as you can that are used to make this bicycle

Metals and alloys Unit 3 Materials and their working properties Uses of metals Answers • A vast range of metals may be used to create a single product Twisted strands of steel in the brake cables, aluminium and steel in the brake handle Frame could be low carbon steel, aluminium alloy Aluminium mud guards Aluminium or stainless steel shopping basket Steel or aluminium spokes Chain made of carbon steel Steel wire in the tyres, zinc in the manufacture of rubber Fused or welded joints are usually brass (a copper / zinc alloy) Steel springs High speed steel in ball bearings, pedals, wheel hubs, headstocks, cranks Aluminium alloy in the pedals

Metals and alloys Unit 3 Materials and their working properties Worksheet • Complete Task 3 of the worksheet

Metals and alloys Unit 3 Materials and their working properties Everything but tin? • Tin probably didn’t feature in your bicycle list • Working with recycled cans is a technique called Kapoaka • Used in Madagascar by small scale workshops, crafters make decorative objects and toys for sale • It has created sustainable employment and helps the poorest in Africa attain a living from the profits

Metals and alloys Unit 3 Materials and their working properties Oxidisation • Non ferrous metals such as copper and bronze, don’t rust, but may oxidise • Oxidising can be caused by corrosion or weather exposure over a period of time • A thin layer of tarnish that appears on the surface of the metal is known as a patina • A natural patina occurring on copper is called Verdigris • The green hue can be manufactured by adding acetic acid to copper and is often used in architecture for aesthetic purposes

Metals and alloys Unit 3 Materials and their working properties Plenary • What are metals commonly extracted from? • Name three ferrous metals • Which metal would you select for high conductivity? • Explain the term ‘alloy’ • Describe the difference between hard and tough • How can you protect metal from oxidisation? • Justify one metal used to manufacture cutting tools

Metals and alloys Unit 3 Materials and their working properties Copyright © 2017 PG Online Limited The contents of this unit are protected by copyright. This unit and all the worksheets, Power. Point presentations, teaching guides and other associated files distributed with it are supplied to you by PG Online Limited under licence and may be used and copied by you only in accordance with the terms of the licence. Except as expressly permitted by the licence, no part of the materials distributed with this unit may be used, reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic or otherwise, without the prior written permission of PG Online Limited. Licence agreement This is a legal agreement between you, the end user, and PG Online Limited. This unit and all the worksheets, Power. Point presentations, teaching guides and other associated files distributed with it is licensed, not sold, to you by PG Online Limited for use under the terms of the licence. The materials distributed with this unit may be freely copied and used by members of a single institution on a single site only. You are not permitted to share in any way any of the materials or part of the materials with any third party, including users on another site or individuals who are members of a separate institution. You acknowledge that the materials must remain with you, the licencing institution, and no part of the materials may be transferred to another institution. You also agree not to procure, authorise, encourage, facilitate or enable any third party to reproduce these materials in whole or in part without the prior permission of PG Online Limited.