Approaching Equilibrium Lesson 1 Approaching Equilibrium Many chemical

Approaching Equilibrium Lesson 1

Approaching Equilibrium Many chemical reactions are reversible if the activation energy is low and the system is closed. Reactants ⇌ Products
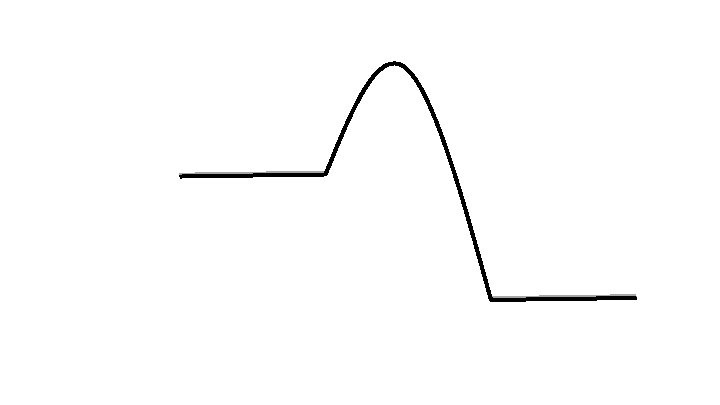
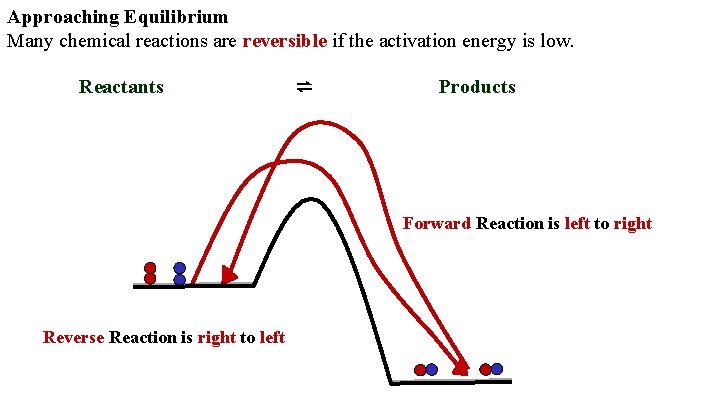
Approaching Equilibrium Many chemical reactions are reversible if the activation energy is low. Reactants ⇌ Products Forward Reaction is left to right Reverse Reaction is right to left

Lets see what happens to some reactants if they are placed in a reaction vessel and allowed to react.

Approaching Equilibrium Products Reactants Forward Rate Reverse Rate

Approaching Equilibrium Products Reactants Forward Rate Reverse Rate

Approaching Equilibrium Products Reactants Forward Rate Reverse Rate

Approaching Equilibrium Products Reactants Forward Rate Reverse Rate

Approaching Equilibrium Products Reactants Forward Rate Reverse Rate
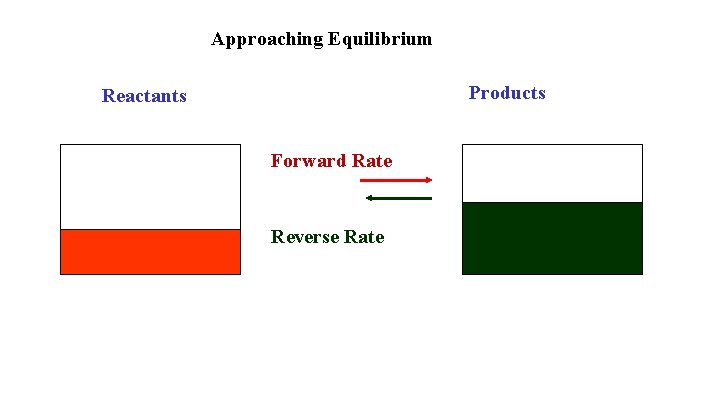
Approaching Equilibrium Products Reactants Forward Rate Reverse Rate
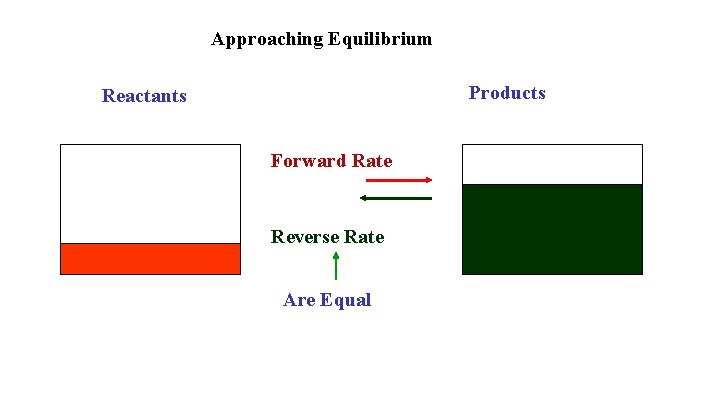
Approaching Equilibrium Products Reactants Forward Rate Reverse Rate Are Equal

Approaching Equilibrium Products Reactants Forward Rate Reverse Rate Are Equal Are Constant

Graphing the Approach to Equilibrium product Concentration reactant Time
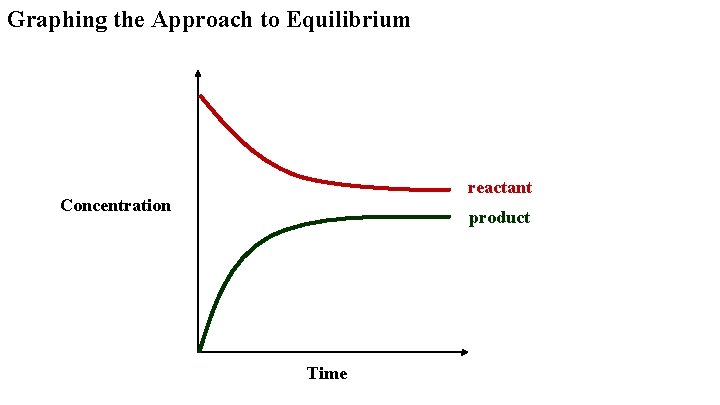
Graphing the Approach to Equilibrium reactant Concentration product Time

Graphing the Approach to Equilibrium Forward rate Concentration Reverse rate Overall rate Time

Approaching Equilibrium Reactant concentrations start high and decrease as the reaction proceeds. The forward rate, which depends on collisions of the reactants, also decreases. Product concentrations start at zero and increase as the reaction proceeds. The reverse rate, which depends on collisions of the products, also increases. Eventually the forward rate is equal to the reverse rate and the concentrations are constant. This is equilibrium.

Characteristics of a System at Equilibrium The Forward rate = The Reverse rate The Reactant and Product concentrations are constant The Macroscopic (observable) properties are constant The system is Dynamic as the forward and reverse reactions continue. The equilibrium can be approached from starting with reactants or starting with products.

Approaching Equilibrium from Products Reactants Forward Rate Reverse Rate
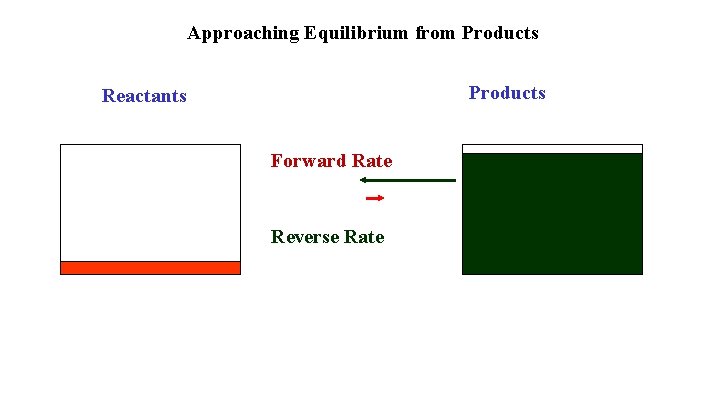
Approaching Equilibrium from Products Reactants Forward Rate Reverse Rate

Approaching Equilibrium from Products Reactants Forward Rate Reverse Rate

Approaching Equilibrium from Products Reactants Forward Rate Reverse Rate

Approaching Equilibrium from Products Reactants Forward Rate Reverse Rate
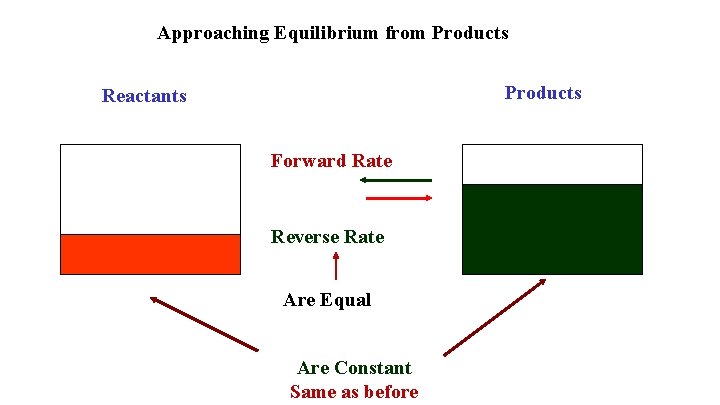
Approaching Equilibrium from Products Reactants Forward Rate Reverse Rate Are Equal Are Constant Same as before

If you start with products all aspects of the approach to equilibrium are reversed Products and Reverse Rate decrease Reactants and Forward Rate increases

Conditions Necessary for Equilibrium Closed system Constant temperature Ea is low so the reaction is reversible
- Slides: 26