APPROACH TO FLUID ELECTROLYTES AND ACID BASE DISORDERS

APPROACH TO FLUID , ELECTROLYTES AND ACID BASE DISORDERS DR. S. SHIVAKUMAR PROF. MEDICINE STANLEY MEDICAL COLLEGE & HOSPITAL

INTRODUCTION: Ø Fluid & Electrolytes can occur as single or multiple disorders ØHyponatremia is a very common disorder ØPottassium disorders can be life threatening ØAcid base disorders common in ICU & medical wards ØChoice of fluid therapy should be appropriate ØTreat individual disorders, but should be combined in multiple disorders.
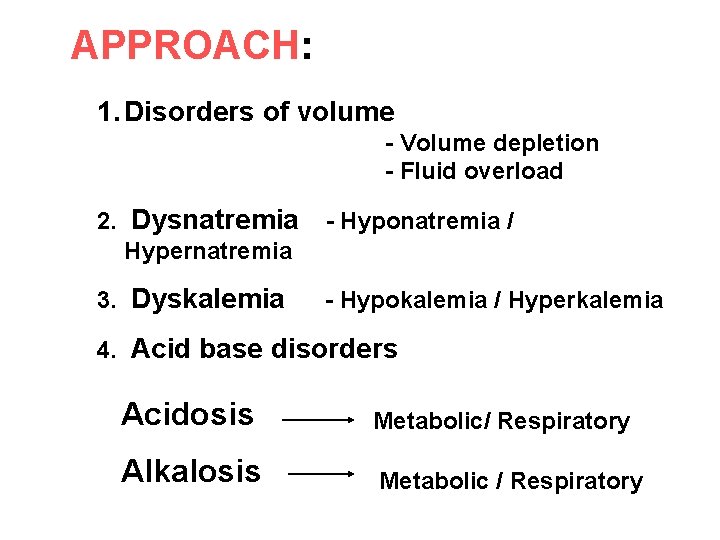
APPROACH: 1. Disorders of volume - Volume depletion - Fluid overload 2. Dysnatremia Hypernatremia - Hyponatremia / 3. Dyskalemia - Hypokalemia / Hyperkalemia 4. Acid base disorders Acidosis Metabolic/ Respiratory Alkalosis Metabolic / Respiratory

CLINICAL APPROACH: CLINICAL FEATURES: Ø Volume depletion ------- Skin turgor loss / Hypotension Ø Volume excess ---- Oedema / HT Ø Hyponatremia/ Hypernatremia ------ CNS Manifestations Ø Hypokalemia ----- Muscle weakness Ø Hyperkalemia ---- Cardiac arrythmias Metabolic acidosis Respiratory alkalosis Hyperventilation

MULTIPLE DISORDERS: Disorder DKA Diarrhea ARF Diuretics Volume Na+ N N N K+ N M. Acidosis + + + - M. Alkalosis - - - +

SINGLE DISORDERS: Disorder SIADH Volume N Na+ Methanol poisoning N N K+ N ABG N Periodic paralysis N N N Acidosis N

FLUID & ELECTROLYTE HOMEOSTASIS 1. Regulation of Volume (Na+) Na+ Aldosterone GFR Renin Renal retention of Na+ Angiotensin Ø ECF Volume is regulated by Na. ( BP & Interstitial volume) 2. Regulation of Osmolality ( Water) H 2 O Osm ADH Kidney retains H 2 O ICF Volume is regulated by osmolarity of plasma. Normal plasma osmolarity = 285 -300 m. Osm/L Calculation= 2(Na + K)+ Sugar/18+Urea/6 Ratio of Na+ to H 2 O decides plasma osmolality. 3. Regulation of Acid Base:
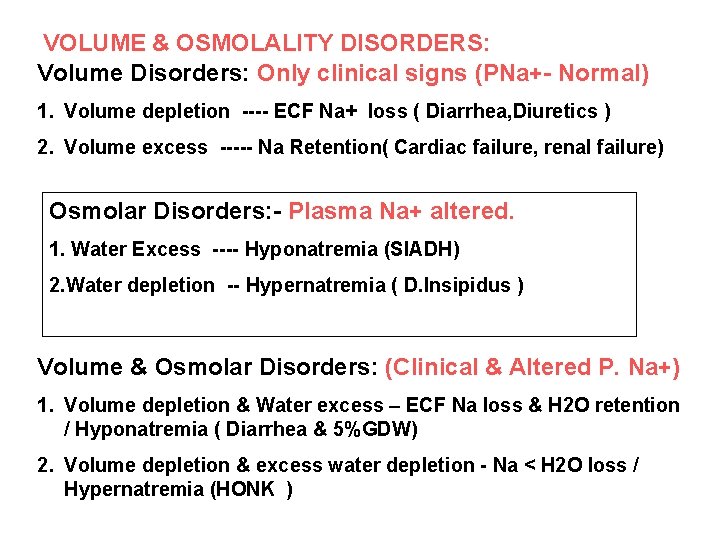
VOLUME & OSMOLALITY DISORDERS: Volume Disorders: Only clinical signs (PNa+- Normal) 1. Volume depletion ---- ECF Na+ loss ( Diarrhea, Diuretics ) 2. Volume excess ----- Na Retention( Cardiac failure, renal failure) Osmolar Disorders: - Plasma Na+ altered. 1. Water Excess ---- Hyponatremia (SIADH) 2. Water depletion -- Hypernatremia ( D. Insipidus ) Volume & Osmolar Disorders: (Clinical & Altered P. Na+) 1. Volume depletion & Water excess – ECF Na loss & H 2 O retention / Hyponatremia ( Diarrhea & 5%GDW) 2. Volume depletion & excess water depletion - Na < H 2 O loss / Hypernatremia (HONK )

Normal 40% (ICF) 20% (ECF) VASCULAR (4%) INTRACELLULAR Interstitial (16%) Total body sodium determines ECF Volume. Plasma Na determines ICF volume. Plasma osmolarity (PNa+) determines fluid movement into cell.
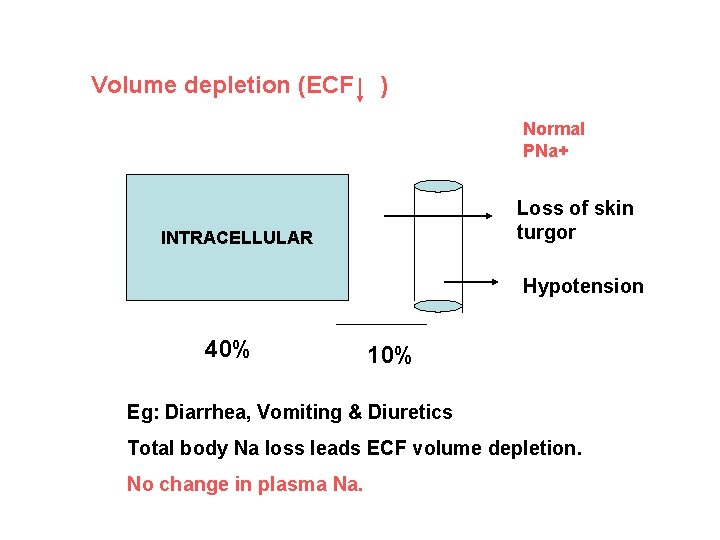
Volume depletion (ECF ) Normal PNa+ Loss of skin turgor INTRACELLULAR Hypotension 40% 10% Eg: Diarrhea, Vomiting & Diuretics Total body Na loss leads ECF volume depletion. No change in plasma Na.

Volume excess (ECF 40% ) 30% Normal PNa+ edema INTRACELLULAR HT Eg: Cardiac failure, Nephrotic syndrome, Renal failure & Cirrhosis Increased total body Na leads to ECF volume excess. Plasma Na normal
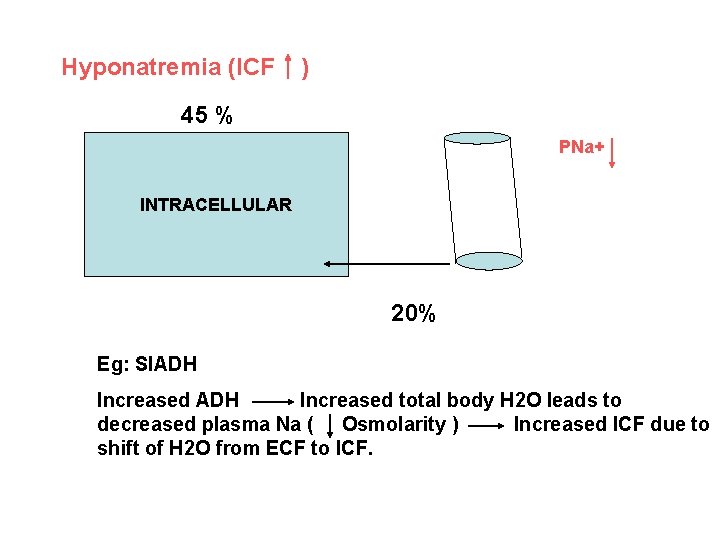
Hyponatremia (ICF ) 45 % PNa+ INTRACELLULAR 20% Eg: SIADH Increased total body H 2 O leads to decreased plasma Na ( Osmolarity ) Increased ICF due to shift of H 2 O from ECF to ICF.
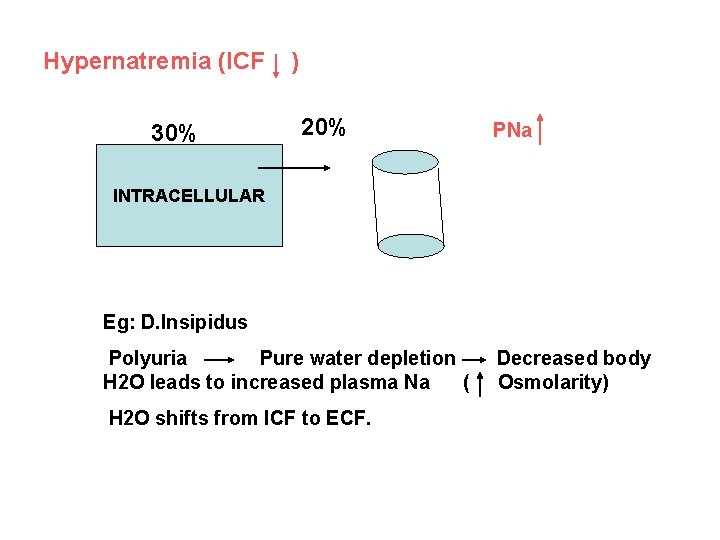
Hypernatremia (ICF 30% ) 20% PNa INTRACELLULAR Eg: D. Insipidus Polyuria Pure water depletion H 2 O leads to increased plasma Na ( H 2 O shifts from ICF to ECF. Decreased body Osmolarity)

Volume depletion & Hyponatremia (ECF & ICF ) 45 % Hypotension, skin turgor loss, Drowsiness H 2 O INTRACELLULAR PNa Na + 10 % Eg: Diarrhea, replaced by 5 % GDW Total body Na decreased by Diarrhea Increasaed H 2 O retention due to 5 % GDW ( Plasma Na low) ECF volume depletion + Increased ICF volume
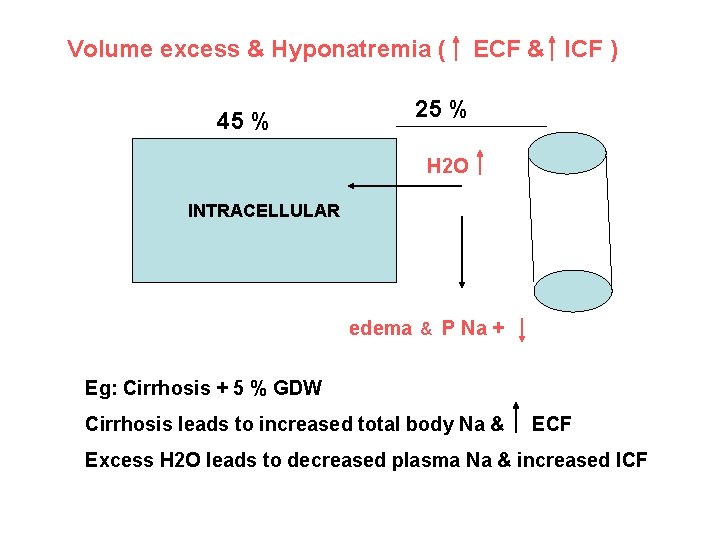
Volume excess & Hyponatremia ( 45 % ECF & ICF ) 25 % H 2 O INTRACELLULAR edema & P Na + Eg: Cirrhosis + 5 % GDW Cirrhosis leads to increased total body Na & ECF Excess H 2 O leads to decreased plasma Na & increased ICF

Volume depletion & Hyperosmolarity ( ECF & 30 % ICF ) 10% Osmolarity INTRACELLULAR Na+ H 2 O Eg: Hyperosmolar Non- Ketotic syndrome ( DM) Polyuria of DM leads to ECF volume depletion ( H 2 O> Na) Increased plasma Glucose leads to hyperosmolarity & ICF depletion Plasma Na can be Normal OR High ( Instead of being low)

Why do we need fluids? FLUIDS --- Healthy individuals ---Regulation of temperature --- loss by sweating ---Excretion of waste products -- Renal / GI 1. 5 – 2. 5 L / Day Salt – 5 gm / day Sick patients: 1. Replacement for losses --- GI losses – Vomiting / Diarrhea --Renal losses -- DKA / Diuretics -- Skin loss – Burns / sweating 2. Maintenance --- for daily requirements
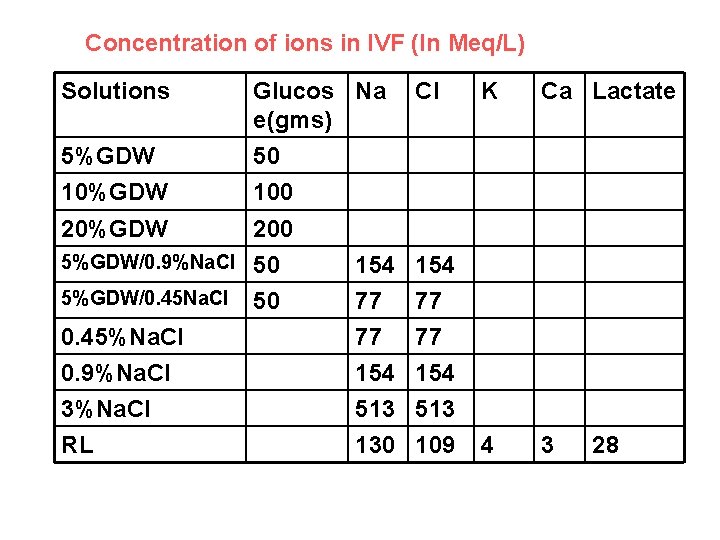
Concentration of ions in IVF (In Meq/L) Solutions 5%GDW 10%GDW Glucos Na e(gms) 50 100 200 5%GDW/0. 9%Na. Cl 50 5%GDW/0. 45 Na. Cl 50 0. 45%Na. Cl 0. 9%Na. Cl 3%Na. Cl RL Cl K Ca Lactate 154 77 77 154 513 109 4 3 20%GDW 154 77 77 154 513 130 28
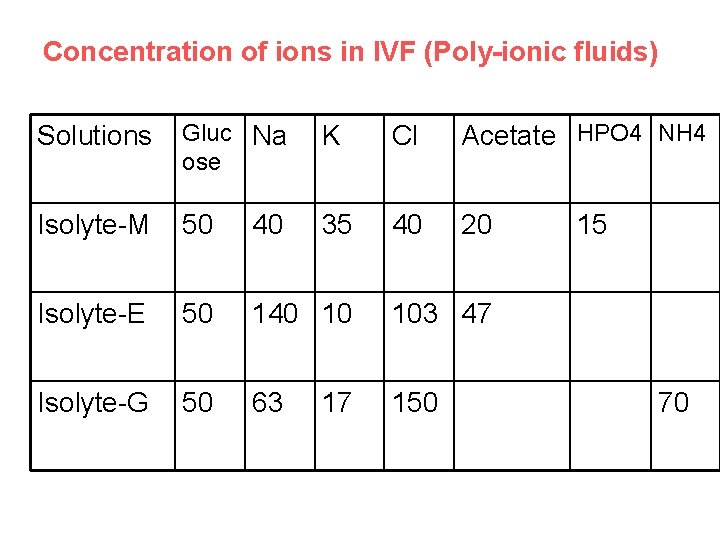
Concentration of ions in IVF (Poly-ionic fluids) Solutions Gluc Na ose K Cl Acetate HPO 4 NH 4 Isolyte-M 50 40 35 40 20 Isolyte-E 50 140 10 103 47 Isolyte-G 50 63 150 17 15 70

DISORDERS OF VOLUME

Volume depletion Hypertonic volume depletion D. Insipidus Isotonic volume depletion Hypotonic volume depletion -Diarrhea -Vomiting HONK Diarrhea replaced by H 2 O DKA Excess sweating PNa-Normal PNa H 2 O > Na loss Na+ = H 2 O loss H 2 O < Na loss

ISOTONIC VOLUME DEPLETION: Causes - vomiting / Diarrhea Assess severity - mild / moderate / severe Body weight: 5 %- mild : skin turgor loss / dry Tongue 5 % - 10 % : Mild + postural hypotension 10 % - severe: moderate + hypotension Correction: Volume depletion: 0 - 8 hrs 0 - 1 hrs --- shock 1 - 8 hrs -- rest of calculated fluids 8 - 24 hrs -- maintanance fluids & concurrent losses Choice of fluids: Diarrhea --- RL Vomiting - 0. 9 % Na. Cl
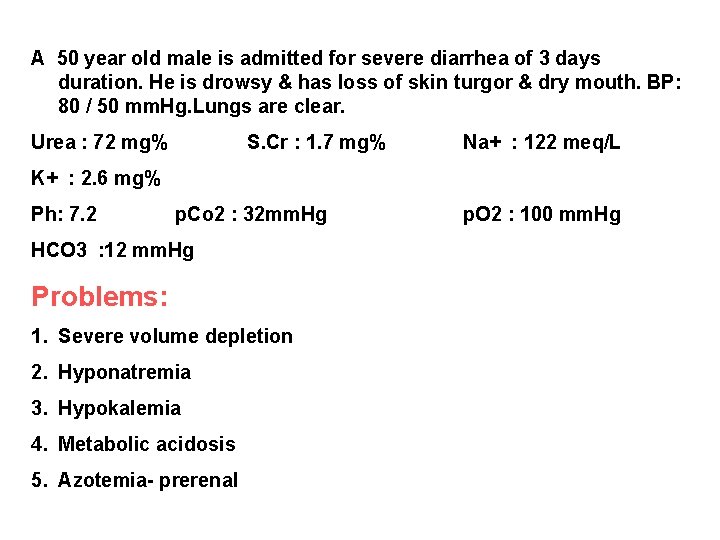
A 50 year old male is admitted for severe diarrhea of 3 days duration. He is drowsy & has loss of skin turgor & dry mouth. BP: 80 / 50 mm. Hg. Lungs are clear. Urea : 72 mg% S. Cr : 1. 7 mg% Na+ : 122 meq/L K+ : 2. 6 mg% Ph: 7. 2 p. Co 2 : 32 mm. Hg HCO 3 : 12 mm. Hg Problems: 1. Severe volume depletion 2. Hyponatremia 3. Hypokalemia 4. Metabolic acidosis 5. Azotemia- prerenal p. O 2 : 100 mm. Hg

50 year Individual ( 50 kg) Severe Volume depletion - 10 % - 5 L Diarrhea – Na, K, HCO 3 loss Replacement fluids - RL 0 -1 hr – 1 L ( Treatment of Shock) 1 - 8 hr – 4 L Maintanence fluid + concurrent loss : 1. 5 – 2. 5 L ( 8 - 24 Hrs) Decreased Na – Corrected by Volume correction RL – Lactate HCO 3 in liver
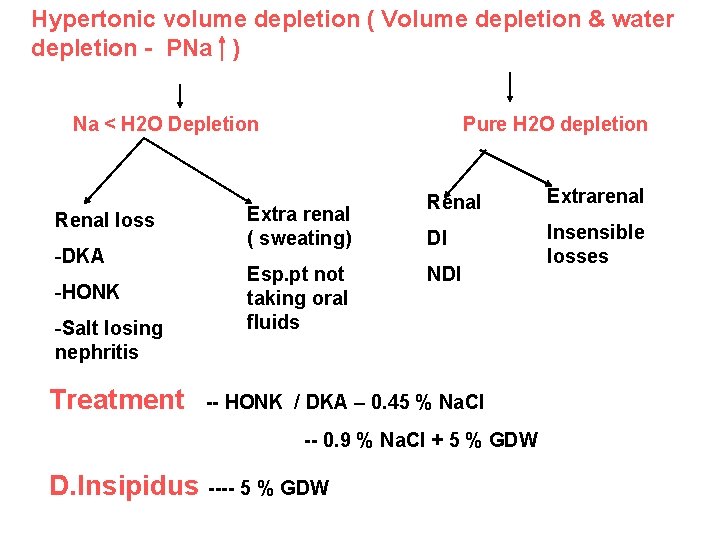
Hypertonic volume depletion ( Volume depletion & water depletion - PNa ) Na < H 2 O Depletion Renal loss -DKA -HONK -Salt losing nephritis Treatment Pure H 2 O depletion Extra renal ( sweating) Esp. pt not taking oral fluids Renal Extrarenal DI Insensible losses NDI -- HONK / DKA – 0. 45 % Na. Cl -- 0. 9 % Na. Cl + 5 % GDW D. Insipidus ---- 5 % GDW

DYSNATREMIAS

Hyponatremia Hypovolemia Euvolemia Hypervolemia Diuretics SIADH CCF Diarrhea Drugs Postoperative CNS & Pulmonary diseases Nephrotic syndrome (Menstruating women) Cirrhosis
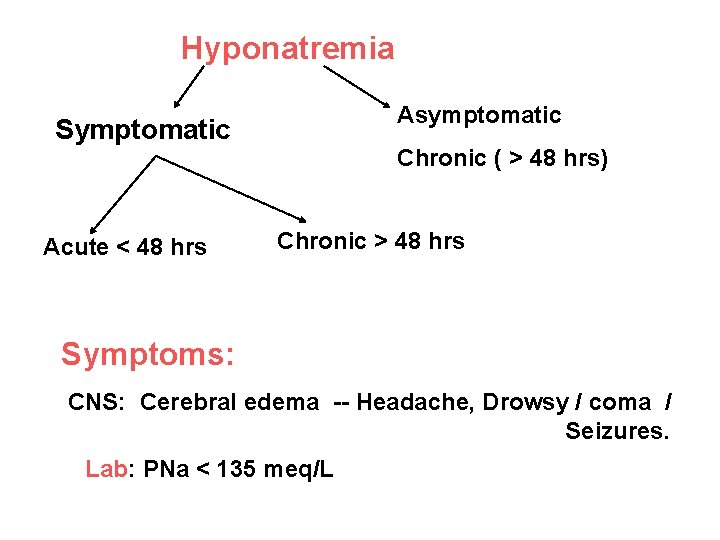
Hyponatremia Asymptomatic Symptomatic Acute < 48 hrs Chronic ( > 48 hrs) Chronic > 48 hrs Symptoms: CNS: Cerebral edema -- Headache, Drowsy / coma / Seizures. Lab: PNa < 135 meq/L

Management Symptomatic Hyponatremia Treatment: Emergency: 3 % Na. Cl 1 - 2 ml/ kg/ hr with co-administration of Furosemide STRATEGY: Removal of H 2 O Furosemide: Natriuresis -- Na + H 2 O Replace Na with 3 % Na. Cl – Excretion of H 2 O Urine output > fluid intake

Asymptomatic Hyponatremia: ( Euvolemia& Hypervolemia) 1. Water restriction : < 1 L / day 2. Demeclocycline -- 300 – 600 mg / day 3. Identify & treat the causes Hypovolemic Hyponatremia: IV Fluids -- Na. Cl / RL Treatment objectives: 1. Gradual correction of PNa 2. Perform serial neurological examination 3. Perform serial Serum Na & Urine Electrolytes & osmolality
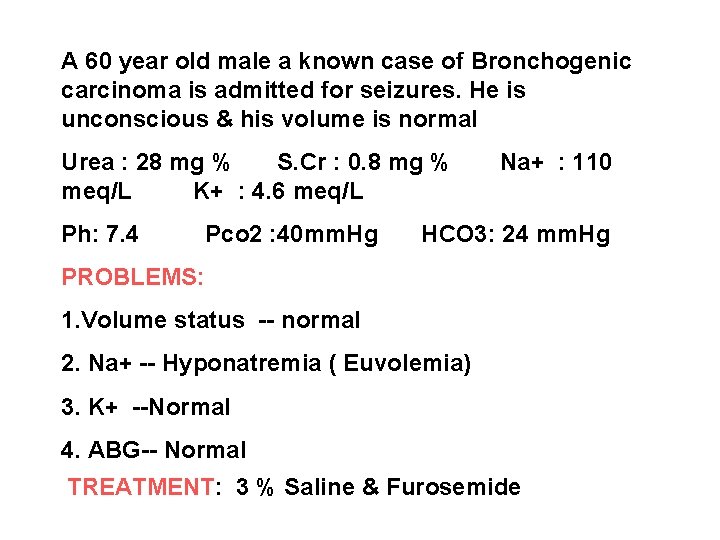
A 60 year old male a known case of Bronchogenic carcinoma is admitted for seizures. He is unconscious & his volume is normal Urea : 28 mg % S. Cr : 0. 8 mg % meq/L K+ : 4. 6 meq/L Ph: 7. 4 Pco 2 : 40 mm. Hg Na+ : 110 HCO 3: 24 mm. Hg PROBLEMS: 1. Volume status -- normal 2. Na+ -- Hyponatremia ( Euvolemia) 3. K+ --Normal 4. ABG-- Normal TREATMENT: 3 % Saline & Furosemide
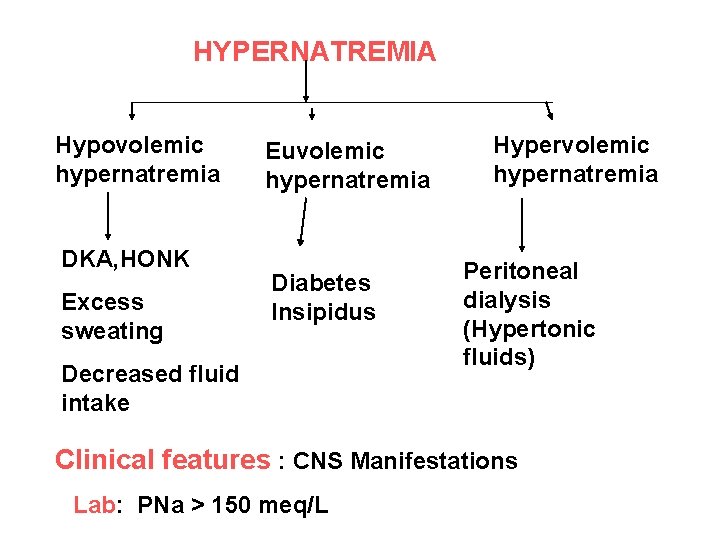
HYPERNATREMIA Hypovolemic hypernatremia DKA, HONK Excess sweating Euvolemic hypernatremia Diabetes Insipidus Decreased fluid intake Hypervolemic hypernatremia Peritoneal dialysis (Hypertonic fluids) Clinical features : CNS Manifestations Lab: PNa > 150 meq/L
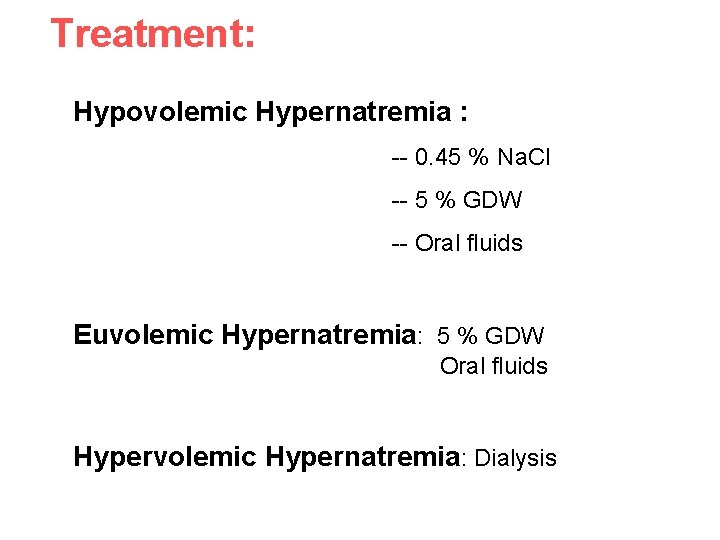
Treatment: Hypovolemic Hypernatremia : -- 0. 45 % Na. Cl -- 5 % GDW -- Oral fluids Euvolemic Hypernatremia: 5 % GDW Oral fluids Hypervolemic Hypernatremia: Dialysis

DYSKALEMIAS
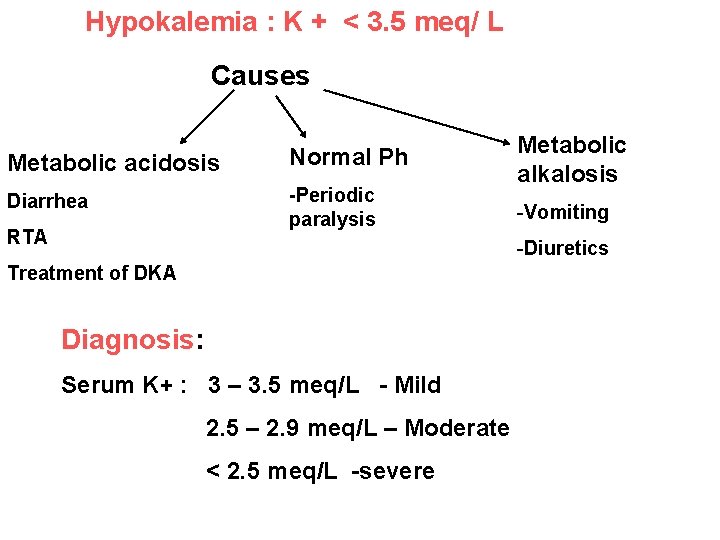
Hypokalemia : K + < 3. 5 meq/ L Causes Metabolic acidosis Normal Ph Diarrhea -Periodic paralysis RTA Metabolic alkalosis -Vomiting -Diuretics Treatment of DKA Diagnosis: Serum K+ : 3 – 3. 5 meq/L - Mild 2. 5 – 2. 9 meq/L – Moderate < 2. 5 meq/L -severe
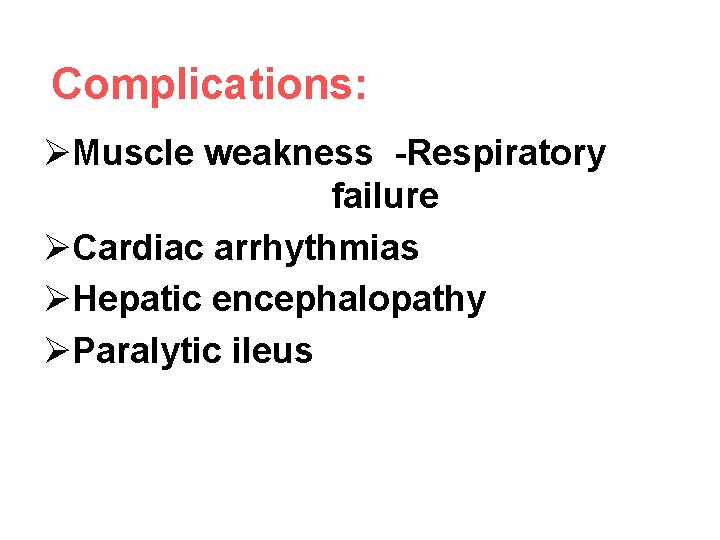
Complications: ØMuscle weakness -Respiratory failure ØCardiac arrhythmias ØHepatic encephalopathy ØParalytic ileus

TREATMENT: IV KCl : 15 % solution 1 cc = 2 meq/L Add to 0. 9 % Na. Cl solution KCl 10 ml = 20 meq/L 20 ml = 40 meq/L Non- urgent situations: Not to exceed 10 meq/hr In urgent situations: 40 meq/Hr Oral solution: 10 % KCl solution ( 15 ml =20 meq/L) --- Diluted in juice 80 – 120 ml / day KCl should be given for 10 – 14 days

HYPERKALEMIA: Causes: Renal failure Drugs -- ACE Inhibitors, Beta-bockers, spironolactone Diagnosis: 1. ECG , 2. Serum K+ Measurement ( > 5 meq/L) Management: 1. Calcium Gluconate: 10 - 30 ml of 10 % solution, acts immediately, action lasts for only 30 mts 2. Insulin- 10 units / Glucose – 50 gms, acts in 15 – 30 Mts, effective for several hours 3. Sodium Bicarbonate: 50 – 150 ml of 7. 5 % Na. HCO 3 4. Cation exchange resins: Na. Polysterene sulphonate (oral/enema) 5. Dialysis

ACID BASE DISORDERS

ABG Disorders: Diagnosis – Ph, HCO 3, Pco 2 1. Check validity 2. Obtain minimal diagnosis 3. Is it a single or mixed ABG disorder 4. Determine Anion Gap 5. Is it a triple ABG Disorder

Obtain minimum diagnosis Ø Look at p. H - Acidosis / Alkalosis Ø Match the Pco 2 or Hco 3 - Metabolic / Respiratory Disorder Primary Change Secondary change Net effect M. Acidosis Hco 3 Pco 2 p. H ( H+) M. Alkalosis Hco 3 Pco 2 p. H ( H+) R. Acidosis Pco 2 Hco 3 p. H ( H+) R. Alkalosis Pco 2 Hco 3 p. H ( H+)
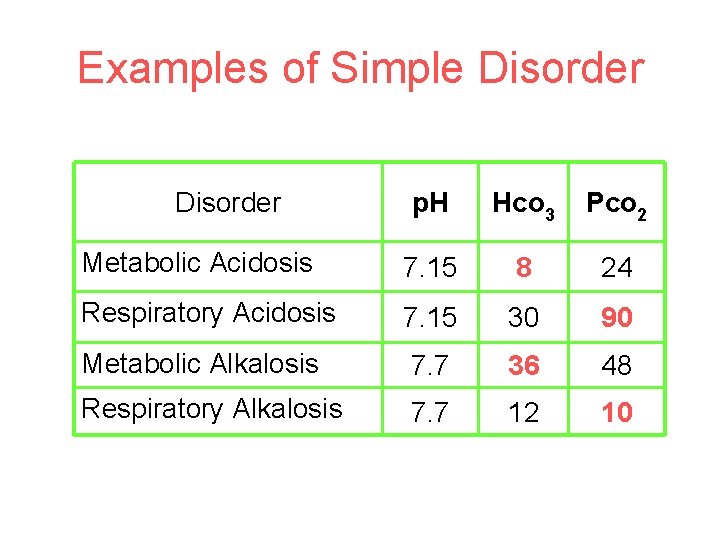
Examples of Simple Disorder p. H Hco 3 Pco 2 Metabolic Acidosis 7. 15 8 24 Respiratory Acidosis 7. 15 30 90 Metabolic Alkalosis 7. 7 36 48 Respiratory Alkalosis 7. 7 12 10

Is it a simple or mixed Acid-base disorder? Simple Disorder : Disorder Metabolic acidosis Metabolic alkalosis Respiratory acidosis Example DKA, Renal failure, Lactic acidosis, Methanol poisoning, Diarrhea, RTA Vomiting, Diuretics, Steroids COPD Respiratory alkalosis Psychogenic hyperventilation, Hepatic Encephalopathy
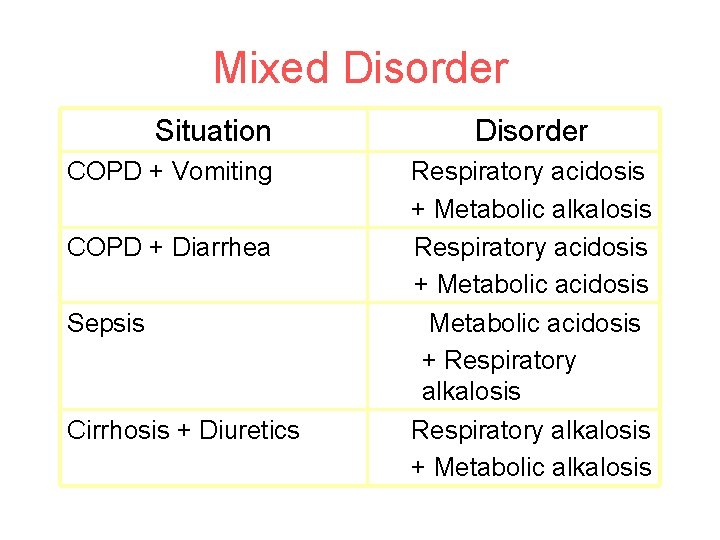
Mixed Disorder Situation COPD + Vomiting COPD + Diarrhea Sepsis Cirrhosis + Diuretics Disorder Respiratory acidosis + Metabolic alkalosis Respiratory acidosis + Metabolic acidosis + Respiratory alkalosis + Metabolic alkalosis
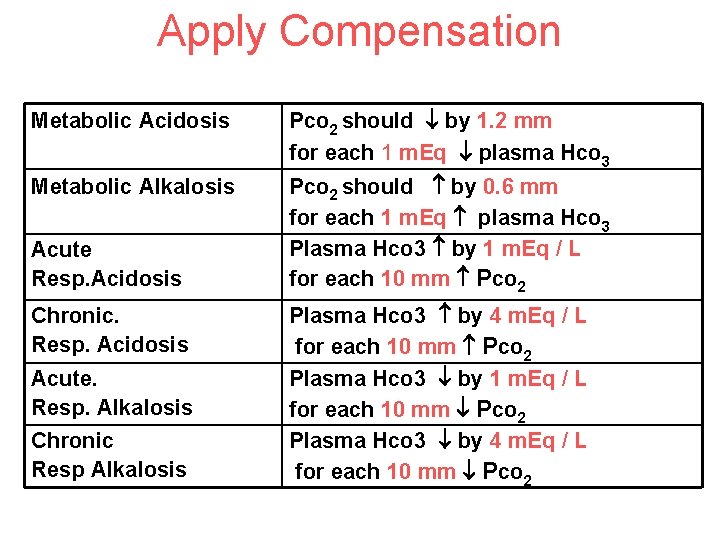
Apply Compensation Metabolic Acidosis Pco 2 should by 1. 2 mm for each 1 m. Eq plasma Hco 3 Metabolic Alkalosis Pco 2 should by 0. 6 mm for each 1 m. Eq plasma Hco 3 Plasma Hco 3 by 1 m. Eq / L for each 10 mm Pco 2 Acute Resp. Acidosis Chronic. Resp. Acidosis Acute. Resp. Alkalosis Chronic Resp Alkalosis Plasma Hco 3 by 4 m. Eq / L for each 10 mm Pco 2 Plasma Hco 3 by 1 m. Eq / L for each 10 mm Pco 2 Plasma Hco 3 by 4 m. Eq / L for each 10 mm Pco 2
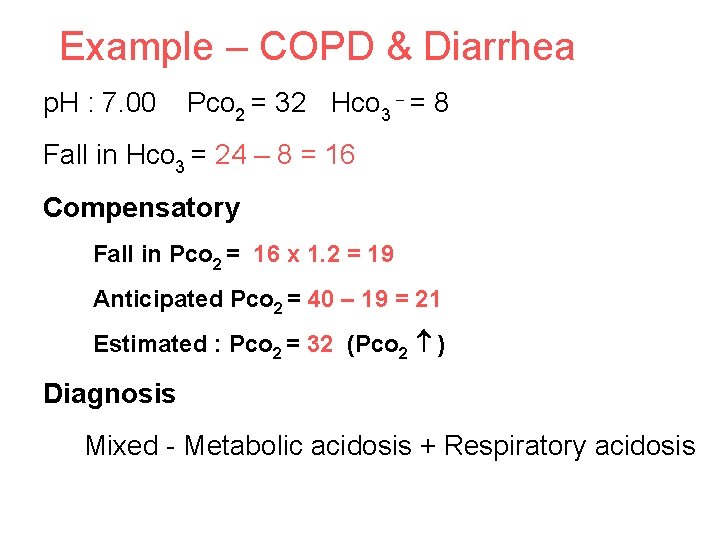
Example – COPD & Diarrhea p. H : 7. 00 Pco 2 = 32 Hco 3 = 8 Fall in Hco 3 = 24 – 8 = 16 Compensatory Fall in Pco 2 = 16 x 1. 2 = 19 Anticipated Pco 2 = 40 – 19 = 21 Estimated : Pco 2 = 32 (Pco 2 ) Diagnosis Mixed - Metabolic acidosis + Respiratory acidosis

Mixed acid base disorder Disorder Compensation Metabolic Acidosis + Respiratory Acidosis eg: COPD + DKA PCo 2 & Hco 3 for simple disturbance Metabolic Alkalosis+ Respiratory Alkalosis eg: Cirrhosis + Diuretics PCo 2 & Hco 3 for simple disturbance PH p. H = PCo 2 Hco 3
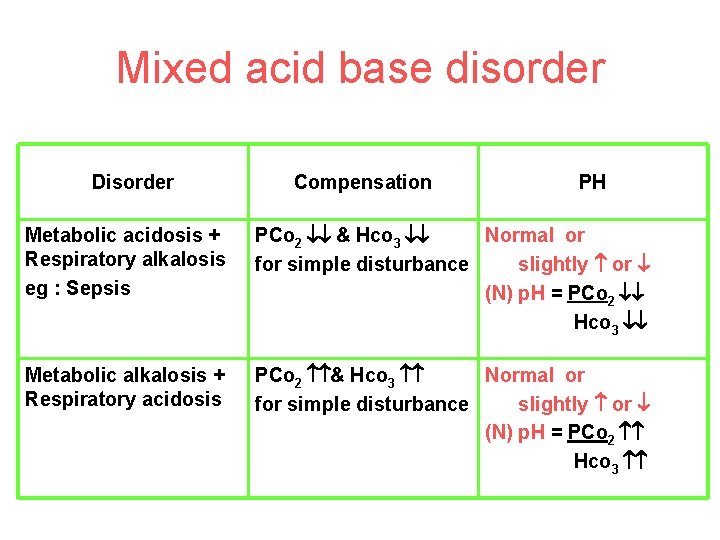
Mixed acid base disorder Disorder Compensation PH Metabolic acidosis + Respiratory alkalosis eg : Sepsis PCo 2 & Hco 3 Normal or for simple disturbance slightly or (N) p. H = PCo 2 Hco 3 Metabolic alkalosis + Respiratory acidosis PCo 2 & Hco 3 Normal or for simple disturbance slightly or (N) p. H = PCo 2 Hco 3
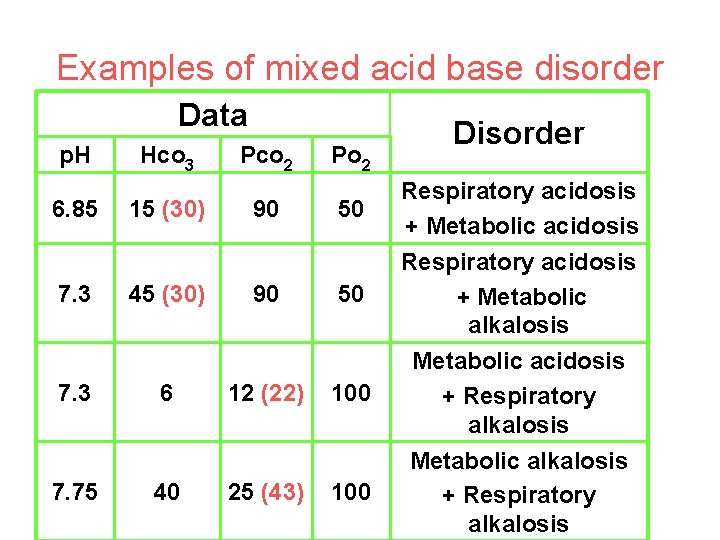
Examples of mixed acid base disorder Data p. H 6. 85 7. 3 7. 75 Hco 3 15 (30) 45 (30) 6 40 Pco 2 90 90 12 (22) 25 (43) Po 2 Disorder 50 Respiratory acidosis + Metabolic acidosis 50 Respiratory acidosis + Metabolic alkalosis 100 Metabolic acidosis + Respiratory alkalosis 100 Metabolic alkalosis + Respiratory alkalosis
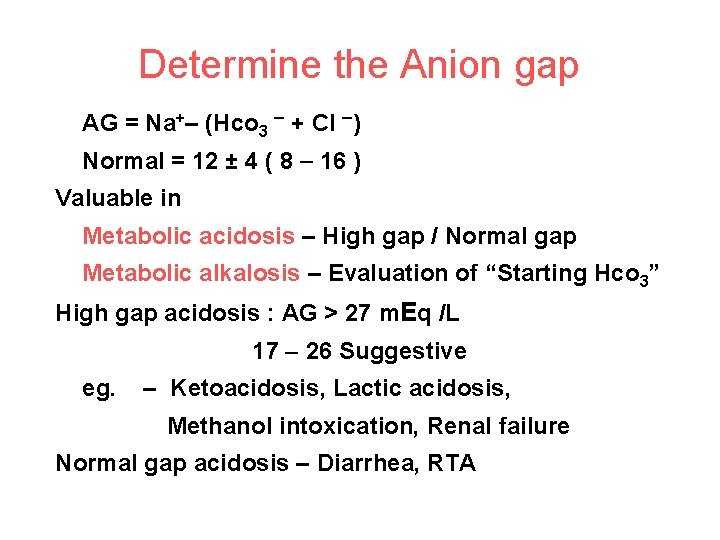
Determine the Anion gap AG = Na+– (Hco 3 + Cl ) Normal = 12 ± 4 ( 8 16 ) Valuable in Metabolic acidosis – High gap / Normal gap Metabolic alkalosis – Evaluation of “Starting Hco 3” High gap acidosis : AG > 27 m. Eq /L 17 26 Suggestive eg. – Ketoacidosis, Lactic acidosis, Methanol intoxication, Renal failure Normal gap acidosis – Diarrhea, RTA
![AG in metabolic alkalosis ] Valuable in diagnosis of Triple disorder (Metabolic acidosis, Met. AG in metabolic alkalosis ] Valuable in diagnosis of Triple disorder (Metabolic acidosis, Met.](http://slidetodoc.com/presentation_image_h/cdbfe3a687a3d44c25255ae3ca058c43/image-51.jpg)
AG in metabolic alkalosis ] Valuable in diagnosis of Triple disorder (Metabolic acidosis, Met. alkalosis & Resp. acidosis) ] Delta ( ) AG = Calculated Anion gap – Normal Anion gap Hco 3 + AG = Starting Hco 3 ] Starting Hco 3 > 29 suggests associated Metabolic Alkalosis in the presence of Metabolic Acidosis

Example Na+ = 135 Hco 3 = 4 cl = 90 p. H = 6. 8 AG = Na ( Hco 3 + cl ) = 135 – (4 + 90) = 41 High gap acidosis AG = Calculated – AG Normal = 41 – 12 = 29 Starting Hco 3 = 4 + 29 = 33 m. Eq / L Starting Hco 3 > 29 suggests associated Metabolic Alkalosis in the presence of Metabolic Acidosis
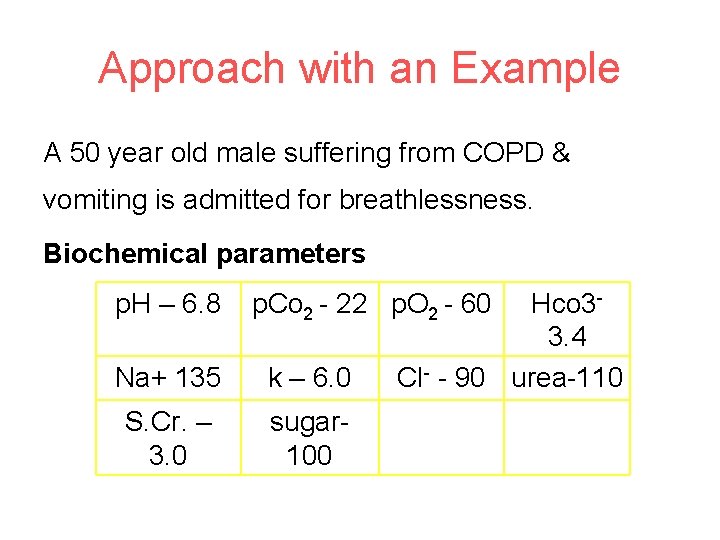
Approach with an Example A 50 year old male suffering from COPD & vomiting is admitted for breathlessness. Biochemical parameters p. H – 6. 8 p. Co 2 - 22 p. O 2 - 60 Na+ 135 k – 6. 0 S. Cr. – 3. 0 sugar 100 Hco 33. 4 Cl- - 90 urea-110

p. H = 6. 8 Pco 2 = 22 Hco 3 = 3. 4 Step 1 : Check Validity H = 24 X Pc 02 = 24 X 22 = 155 n. Eq / L = p. H(6. 8) HCo 3 3. 4 Step 2: Obtain minimum diagnosis p. H = 6. 8 Pco 2 = 22 Hco 3 = 3. 4 Metabolic acidosis
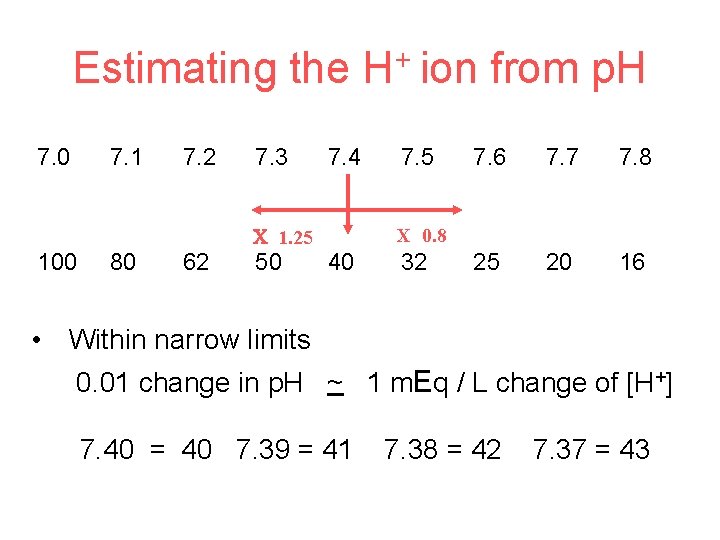
Estimating the H+ ion from p. H 7. 0 7. 1 7. 2 7. 3 7. 4 1. 25 100 80 62 50 7. 5 7. 6 7. 7 7. 8 25 20 16 X 0. 8 40 32 • Within narrow limits 0. 01 change in p. H ~ 1 m. Eq / L change of [H+] 7. 40 = 40 7. 39 = 41 7. 38 = 42 7. 37 = 43

p. H = 6. 8 Pco 2 = 22 Hco 3 = 3. 4 Step 3: Is it a Simple or Mixed Acid base disturbance By applying compensation (24 3. 4 = 20. 6 ; 20. 6 1. 2 = 24. 7; 40 24. 7 = 15. 3) Predicted Pco 2 = 15. 3, but Observed Pco 2 = 22 Mixed disorder Metabolic Acidosis + Respiratory Acidosis

p. H = 6. 8 Pco 2 = 22 Hco 3 = 3. 4 Step 4: Determine Anion Gap Na = 135 Cl = 90 Hco 3 = 3. 4 AG = 135 – (3. 4 + 90) = 41. 6 ] High Gap acidosis
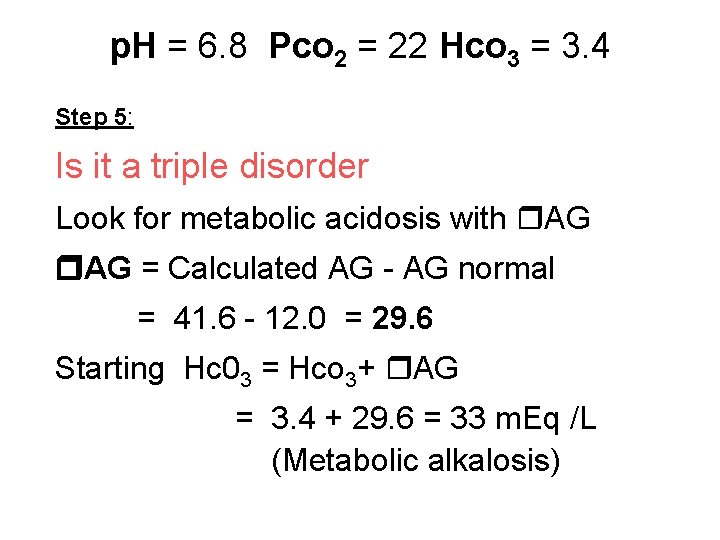
p. H = 6. 8 Pco 2 = 22 Hco 3 = 3. 4 Step 5: Is it a triple disorder Look for metabolic acidosis with AG AG = Calculated AG - AG normal = 41. 6 - 12. 0 = 29. 6 Starting Hc 03 = Hco 3+ AG = 3. 4 + 29. 6 = 33 m. Eq /L (Metabolic alkalosis)
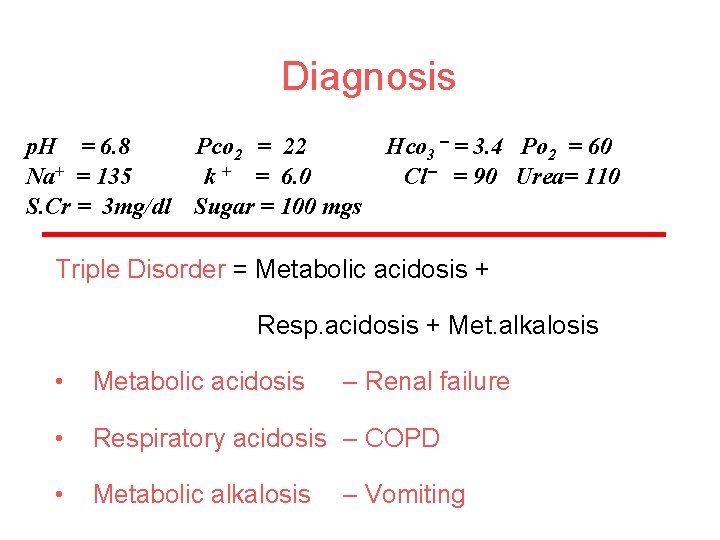
Diagnosis p. H = 6. 8 Pco 2 = 22 Na+ = 135 k + = 6. 0 S. Cr = 3 mg/dl Sugar = 100 mgs Hco 3 = 3. 4 Po 2 = 60 Cl = 90 Urea= 110 Triple Disorder = Metabolic acidosis + Resp. acidosis + Met. alkalosis • Metabolic acidosis • Respiratory acidosis – COPD • Metabolic alkalosis – Renal failure – Vomiting
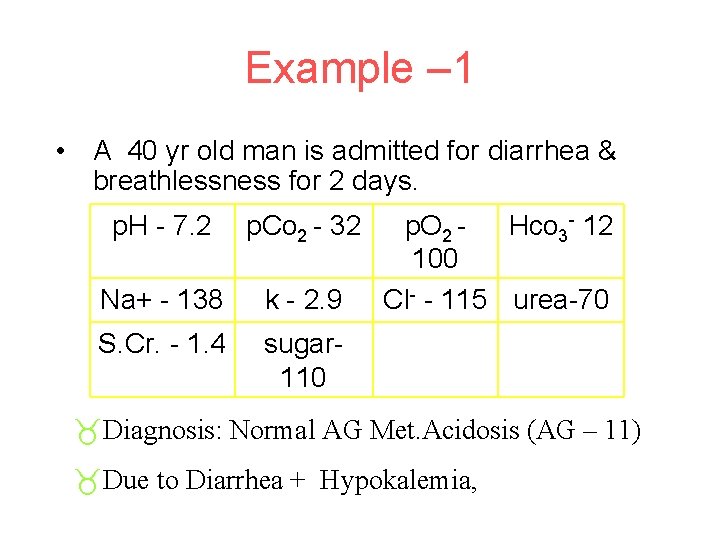
Example – 1 • A 40 yr old man is admitted for diarrhea & breathlessness for 2 days. p. H - 7. 2 p. Co 2 - 32 Na+ - 138 k - 2. 9 S. Cr. - 1. 4 sugar 110 p. O 2 Hco 3 - 12 100 Cl- - 115 urea-70 _Diagnosis: Normal AG Met. Acidosis (AG – 11) _Due to Diarrhea + Hypokalemia,

Example – 2 • A 40 yr old man is admitted for diarrhea of 1 week & breathlesness of 1 day p. H - 7. 1 p. Co 2 - 20 Na+ - 140 k + - 6. 9 S. Cr. - 5. 4 sugar 110 p. O 2 Hco 3 - 6 100 Cl- - 105 urea-120 _Diagnosis: High gap Acidosis (AG - 29) _Due to Renal failure + Hyperkalemia
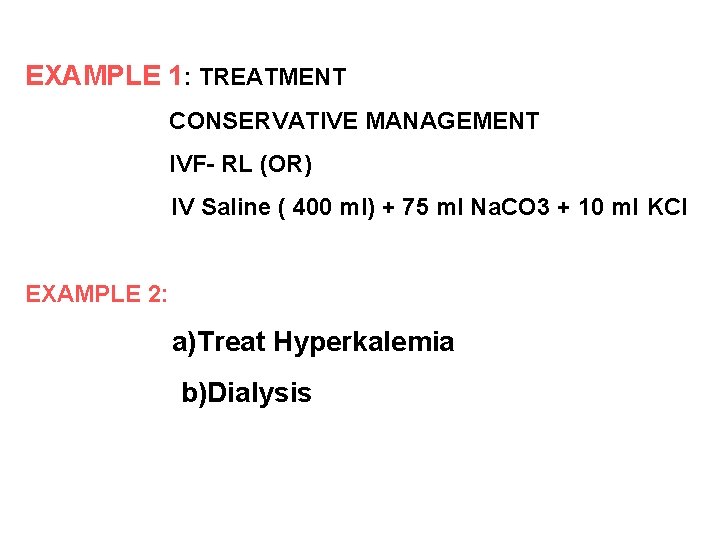
EXAMPLE 1: TREATMENT CONSERVATIVE MANAGEMENT IVF- RL (OR) IV Saline ( 400 ml) + 75 ml Na. CO 3 + 10 ml KCl EXAMPLE 2: a)Treat Hyperkalemia b)Dialysis
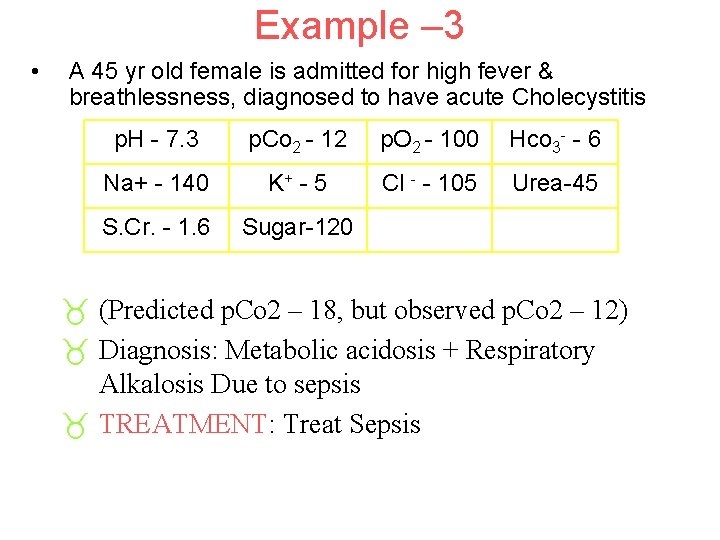
Example – 3 • A 45 yr old female is admitted for high fever & breathlessness, diagnosed to have acute Cholecystitis p. H - 7. 3 p. Co 2 - 12 p. O 2 - 100 Hco 3 - - 6 Na+ - 140 K+ - 5 Cl - - 105 Urea-45 S. Cr. - 1. 6 Sugar-120 _ (Predicted p. Co 2 – 18, but observed p. Co 2 – 12) _ Diagnosis: Metabolic acidosis + Respiratory Alkalosis Due to sepsis _ TREATMENT: Treat Sepsis
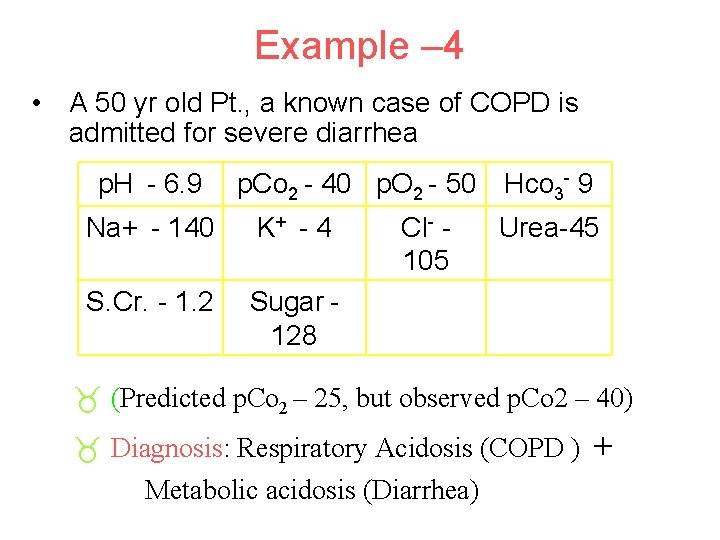
Example – 4 • A 50 yr old Pt. , a known case of COPD is admitted for severe diarrhea p. H - 6. 9 p. Co 2 - 40 p. O 2 - 50 Hco 3 - 9 Na+ - 140 K+ - 4 S. Cr. - 1. 2 Sugar 128 Cl- 105 Urea-45 _ (Predicted p. Co 2 – 25, but observed p. Co 2 – 40) _ Diagnosis: Respiratory Acidosis (COPD ) Metabolic acidosis (Diarrhea) +
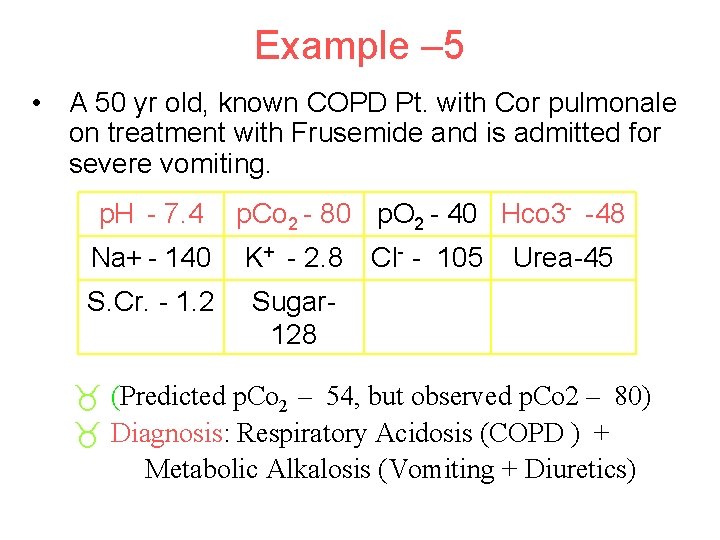
Example – 5 • A 50 yr old, known COPD Pt. with Cor pulmonale on treatment with Frusemide and is admitted for severe vomiting. p. H - 7. 4 p. Co 2 - 80 p. O 2 - 40 Hco 3 - -48 Na+ - 140 K+ - 2. 8 S. Cr. - 1. 2 Sugar 128 Cl- - 105 Urea-45 _ (Predicted p. Co 2 – 54, but observed p. Co 2 – 80) _ Diagnosis: Respiratory Acidosis (COPD ) + Metabolic Alkalosis (Vomiting + Diuretics)
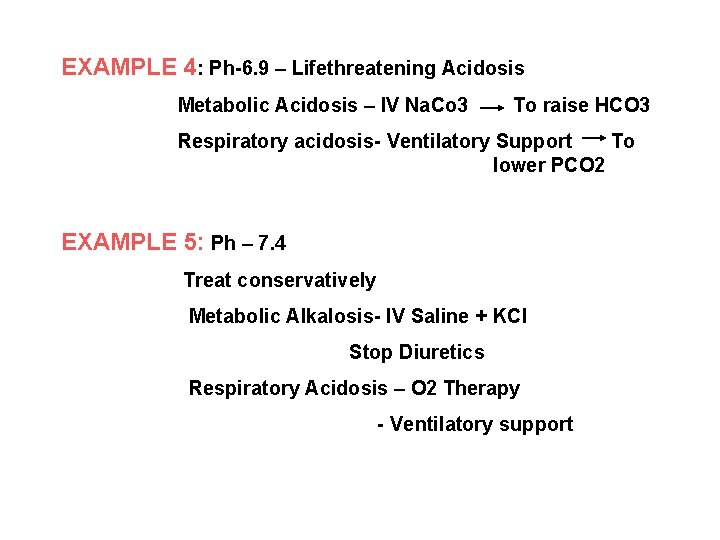
EXAMPLE 4: Ph-6. 9 – Lifethreatening Acidosis Metabolic Acidosis – IV Na. Co 3 To raise HCO 3 Respiratory acidosis- Ventilatory Support To lower PCO 2 EXAMPLE 5: Ph – 7. 4 Treat conservatively Metabolic Alkalosis- IV Saline + KCl Stop Diuretics Respiratory Acidosis – O 2 Therapy - Ventilatory support
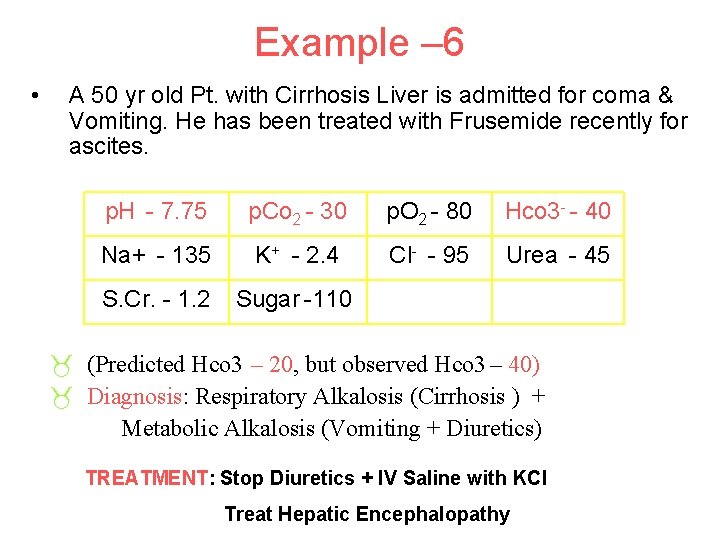
Example – 6 • A 50 yr old Pt. with Cirrhosis Liver is admitted for coma & Vomiting. He has been treated with Frusemide recently for ascites. p. H - 7. 75 p. Co 2 - 30 p. O 2 - 80 Hco 3 - - 40 Na+ - 135 K+ - 2. 4 Cl- - 95 Urea - 45 S. Cr. - 1. 2 Sugar -110 _ (Predicted Hco 3 – 20, but observed Hco 3 – 40) _ Diagnosis: Respiratory Alkalosis (Cirrhosis ) + Metabolic Alkalosis (Vomiting + Diuretics) TREATMENT: Stop Diuretics + IV Saline with KCl Treat Hepatic Encephalopathy

Summary • Suspect the diagnosis from history • Suspect the disturbance from physical symptoms • Assess volume status to determine fluid therapy • Determine P. Sodium to determine water intake • Measure K+ levels & Utilize ECG to determine cardiac effects

• Evaluate routine laboratory date : Sugar, RFT, LFT, Na+, K+ • Establish the cause of Acid Base disorder (Utilize thoughtful differential diagnosis) • Direct management of underlying disorder, unless p. H is in a dangerous range

- Slides: 70