Applied Hydrobiology Sources and types of pollution Water

Applied Hydrobiology Sources and types of pollution

Water pollution: �Holdgate (1971) defined pollution as something that is present in the wrong place, wrong time and wrong quantity. �The legal definition of water pollution-Pollution arises by the addition of something to water that changes its natural qualities (Wisdom 1956)

Complete definition �The introduction by man into the environment of substances or energy, liable to cause hazards to human health, harm to living sources and ecological systems, damage to structure and amenity or interference with the legitimate use of the environment Holdgate 1979).

What are pollutants? �Acids and Alkalis �Anions (e. g sulphides, sulphites, cyanide) �Detergents �Domestic sewage and farm manures �Food processing wastes (including farm processes) �Gases (chlorine and ammonia) �Heat

What are pollutants? �Metals �Nutrients (phosphates and nitrates) �Oil and oil dispersants) �Organic toxic wastes (phenols and formaldehyde) �Pathogens �Pesticides �Polychlorinated biphenyls �Radionuclides

General effects �Acute-large dose short duration �Chronic-small dose over long period
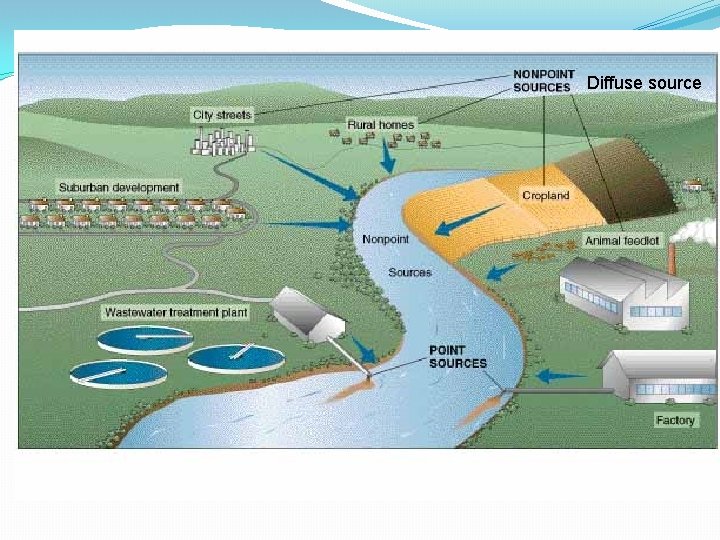
Diffuse source

Sources of pollution Point sources �Waste water or effluent is discharged into water sources at a particular point. �e. g. sewage through a pipe �Most effluents are point sources

Three types of sewerage system �Foul sewers �Surface water or storm sewers �Combined systems �Some industries treat their own waste but have to meet consent criteria, that depend on what the effluent is being discharged into

Standards � 30/20 sewage effluent should contain no more than 30 mg l-1 suspended solids and BOD should have a value not exceeding 20 mg l-1 �In trout and salmon rivers this reduced to 15/10 �Ammonia is set at 5 mg l-1 or 1 mg l-1 depending on the receiving water
Oxygen and Water �What else can affect the amount of O 2 in the water? �Temperature �Speed of water flow �Roughness of surface over which water flows

More Examples: Oxygen and Water �Biochemical Oxygen Demand – What does this mean? �Anything in the water that bacteria can break down. �Bacteria will use up oxygen in the water �Other aerobic organisms will die

Sources of pollution Diffuse sources �The hardest to combat. Water run off from agricultural land, the hydrological cycle is contaminated in a diffuse way

Pollutants Inert suspensions �Mineral particulate �Suspension of minerals in the water �Comes usually from mining quarrying �Changes the state of the river bed which in turn changes aquatic habitats
Poisons and toxins �Acids and alkalis � usual range p. H 6 -8 �Associated with industry and acid rain �Toxicity of ammonia, cyanide and some heavy metals can increase at lower p. H
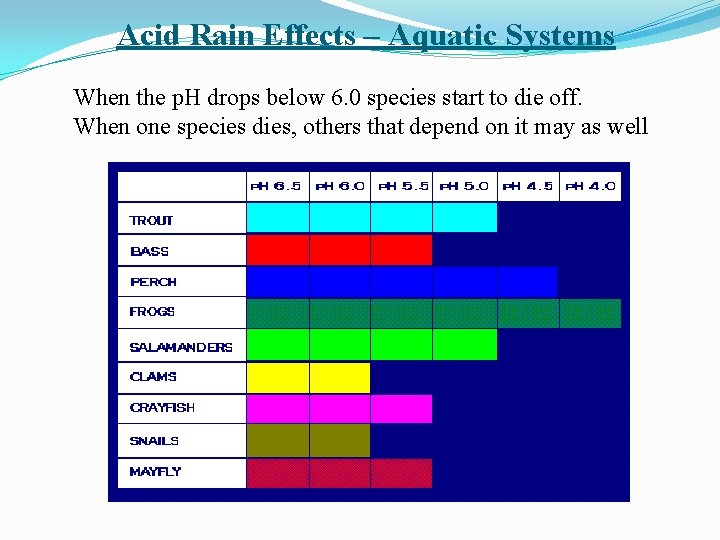
Acid Rain Effects – Aquatic Systems When the p. H drops below 6. 0 species start to die off. When one species dies, others that depend on it may as well

Heavy metals �Mining, quarrying, natural disturbance �Zinc, copper, lead, cadmium, mercury, chromium, nickel and aluminium

Organic chemical residues �Used in the manufacture of pesticides �Include-organochlorides, organophosphates, pyrethroids, phenols, herbicides, polychlorinated biphenyls (PCBs) �The Environment Agency has a red list �Many endocrine disrupters

Gases �Ammonia �Methane �Released by anaerobic breakdown �Ammonia very toxic to fish

Inorganic reducing agents �Sulphides, sulphates, ferric compounds �Increase oxygen demand exerted on the water

Biodegradable organic matter �Plant and animal origin �Increases BOD

Inorganic materials �Nitrates, phosphates �Stimulate primary productivity �Algal blooms

Oils �Usually accidental spillage �Quarter of incidents occur in fresh water �Impose high BOD
Detergents �Synthetic �Foaming �Some similar effects as oil

Heat �Power stations use water for cooling �Stimulates biolgical processes

Biological agents �Chemicals that affect biological systems �Carcinogens, radioactive chemicals

Tame survey �Survey of the chemical and biological effects of pollution on a river
- Slides: 27