Applied Groundwater Hydrology HWR 412 First term 2010













- Slides: 29

Applied Groundwater Hydrology HWR 412 First term 2010 -2011 (14311432) Dr. Jarbou Bahrawi

Topics to be Covered • • • Review of Groundwater Terminology Groundwater Chemistry Transport of Pollution in Aquifers. Well Hydraulics. Groundwater Models.

Chapter 2 Groundwater Chemistry

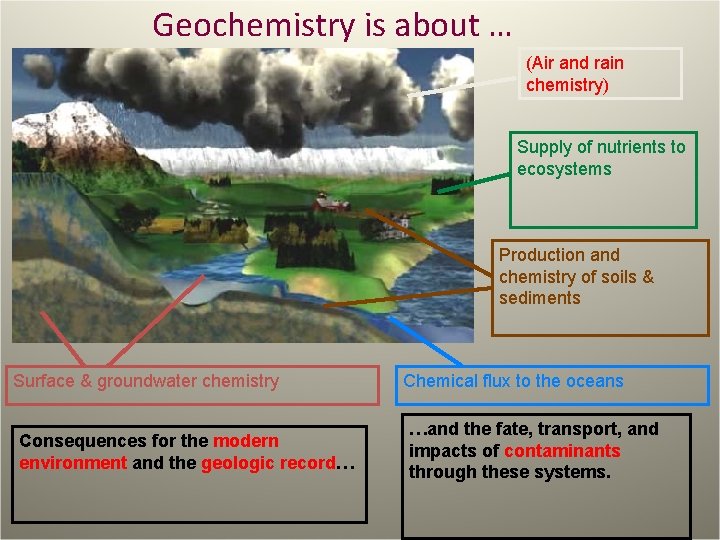
Geochemistry is about … (Air and rain chemistry) Supply of nutrients to ecosystems Production and chemistry of soils & sediments Surface & groundwater chemistry Consequences for the modern environment and the geologic record… Chemical flux to the oceans …and the fate, transport, and impacts of contaminants through these systems.

Periodic Table

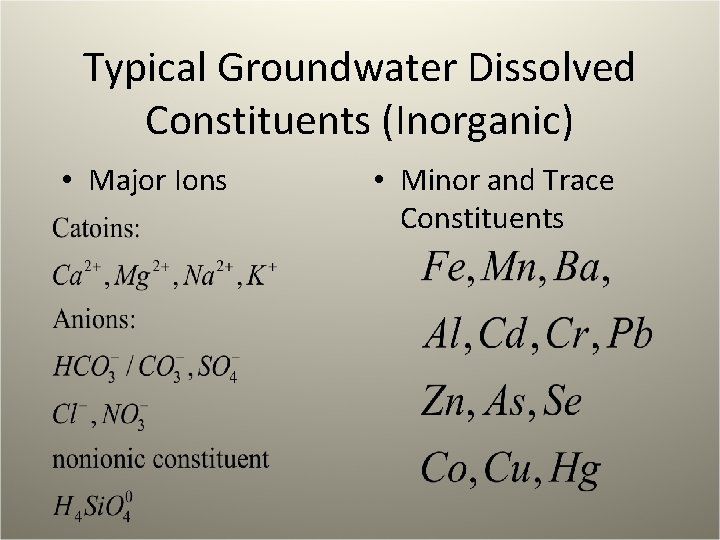
Typical Groundwater Dissolved Constituents (Inorganic) • Major Ions • Minor and Trace Constituents

Water Chemistry • Groundwater is never pure, rather, it contains small amounts of dissolved gases and solids.

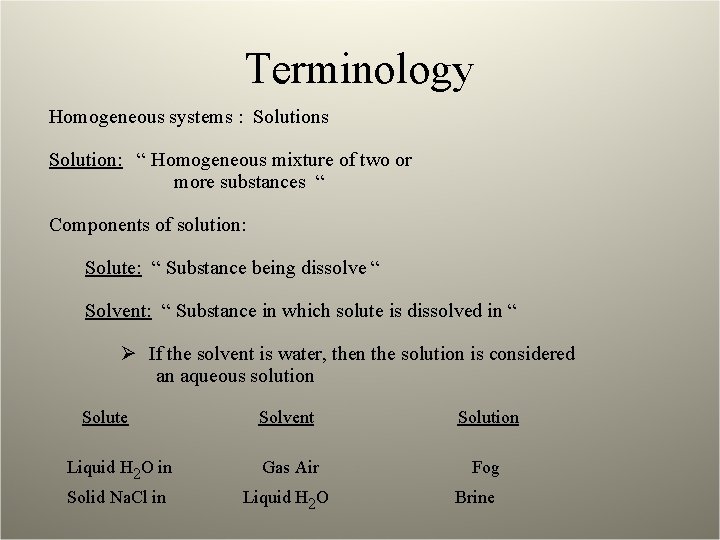
Terminology Homogeneous systems : Solutions Solution: “ Homogeneous mixture of two or more substances “ Components of solution: Solute: “ Substance being dissolve “ Solvent: “ Substance in which solute is dissolved in “ Ø If the solvent is water, then the solution is considered an aqueous solution Solute Solvent Solution Liquid H 2 O in Gas Air Fog Solid Na. Cl in Liquid H 2 O Brine

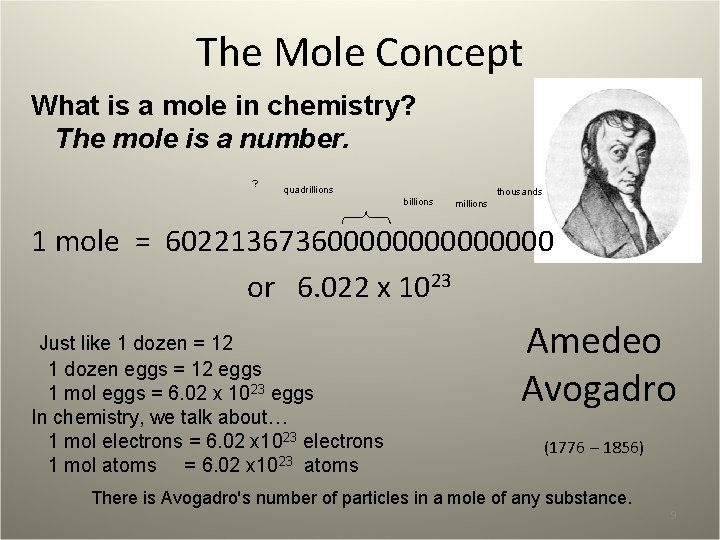
The Mole Concept What is a mole in chemistry? The mole is a number. ? quadrillions billions thousands millions 1 mole = 60221367360000000 or 6. 022 x 1023 Just like 1 dozen = 12 1 dozen eggs = 12 eggs 1 mol eggs = 6. 02 x 1023 eggs In chemistry, we talk about… 1 mol electrons = 6. 02 x 1023 electrons 1 mol atoms = 6. 02 x 1023 atoms Amedeo Avogadro (1776 – 1856) There is Avogadro's number of particles in a mole of any substance. 9

Why do we have to talk about moles? • Atoms and molecules are too small to see or handle in the lab. • We must talk about quantities large enough to see and touch. • We cannot weigh out 1 atom of Ag. • We can weigh out 1 mol of Ag (6. 02 x 1023 Ag atoms). Why does the mole have such a weird number? In the periodic table, 47 Ag 107. 87 1 atom of Ag is 107. 87 amu 1 mol of Ag (6. 02 x 1023 Ag atoms) weigh 107. 87 g. We can make use of all the atomic masses on the periodic table in this manner. H weighs 1. 008 amu/H atom, 1. 008 g/mole H N weighs 14. 01 amu/N atom, 14. 01 g/mole N. 10

Be sure you learn the meaning of the word “molar” when used in front of another word. “molar” means “per mole” “molar mass” means “mass per mole” = “mass of one mole” REMEMBER!!!: molar mass has units of g/mole 11

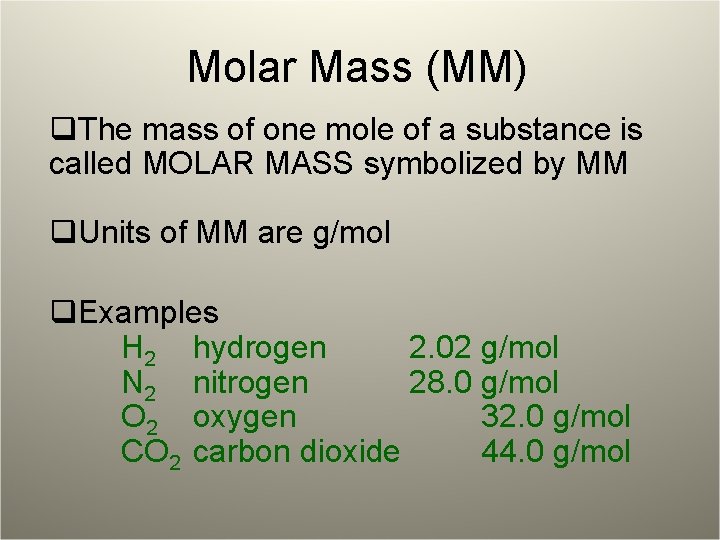
Molar Mass (MM) q. The mass of one mole of a substance is called MOLAR MASS symbolized by MM q. Units of MM are g/mol q. Examples H 2 hydrogen 2. 02 N 2 nitrogen 28. 0 O 2 oxygen CO 2 carbon dioxide g/mol 32. 0 g/mol 44. 0 g/mol

Mole Example • One Mole of a chemical species is its formula weight in grams, calculated by adding up the atomic weights of all the atoms in that species. • E. g. Na. Cl = Na (at. wt. 22. 991) + Cl (at. wt. 35. 457) = 58. 448 gm.

1. Calculate the MM of CO 2. Show your setup. Don’t forget your units! MM(CO 2)= 12. 01 + 2(16. 00) = 44. 01 g/mol 2. What is the molecular weight of CO 2? Ans. 44. 01 amu/CO 2 molecule 14

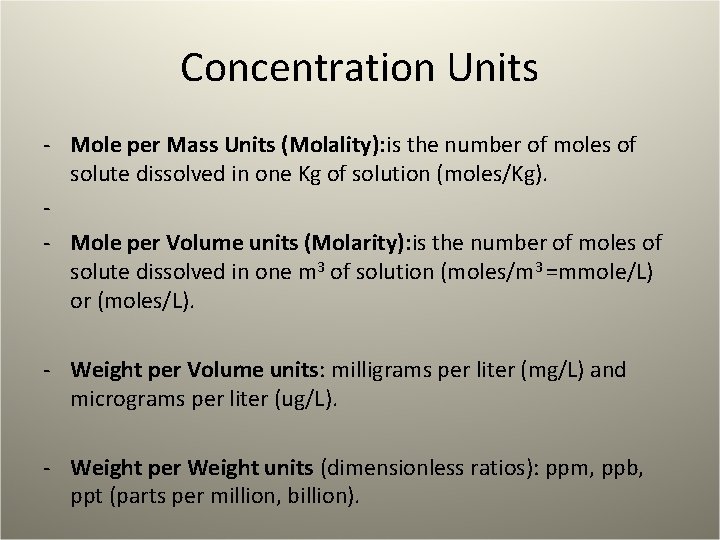
Concentration Units - Mole per Mass Units (Molality): is the number of moles of solute dissolved in one Kg of solution (moles/Kg). - - Mole per Volume units (Molarity): is the number of moles of solute dissolved in one m 3 of solution (moles/m 3 =mmole/L) or (moles/L). - Weight per Volume units: milligrams per liter (mg/L) and micrograms per liter (ug/L). - Weight per Weight units (dimensionless ratios): ppm, ppb, ppt (parts per million, billion).

Formulas for Units

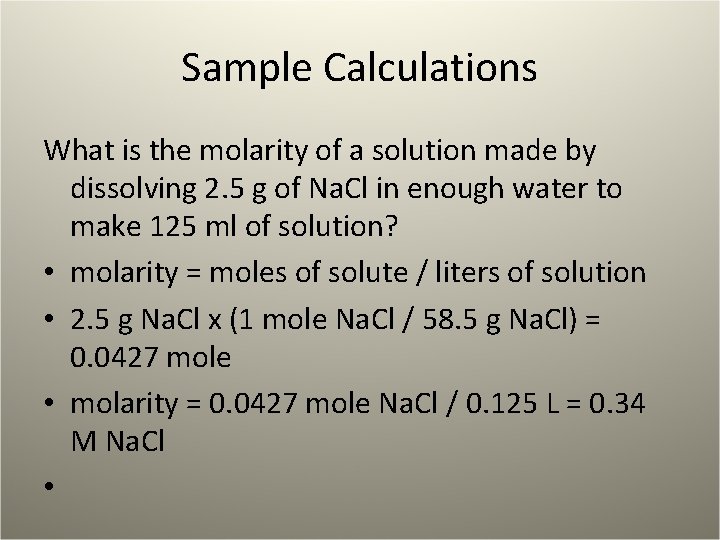
Sample Calculations What is the molarity of a solution made by dissolving 2. 5 g of Na. Cl in enough water to make 125 ml of solution? • molarity = moles of solute / liters of solution • 2. 5 g Na. Cl x (1 mole Na. Cl / 58. 5 g Na. Cl) = 0. 0427 mole • molarity = 0. 0427 mole Na. Cl / 0. 125 L = 0. 34 M Na. Cl •

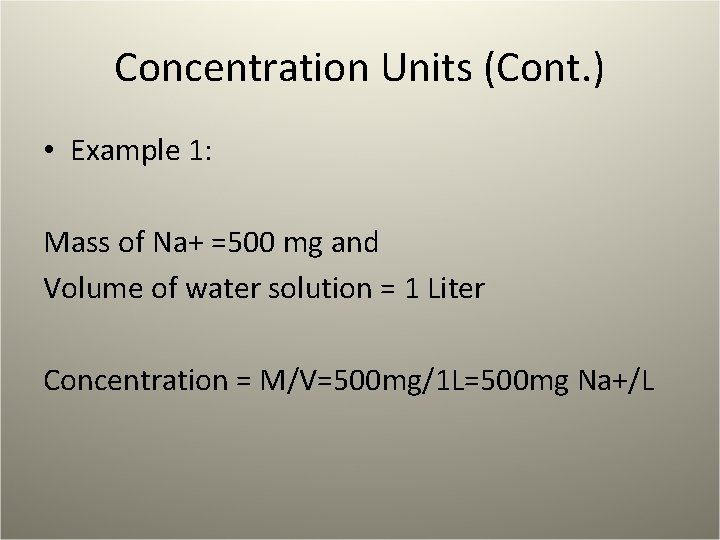
Concentration Units (Cont. ) • Example 1: Mass of Na+ =500 mg and Volume of water solution = 1 Liter Concentration = M/V=500 mg/1 L=500 mg Na+/L

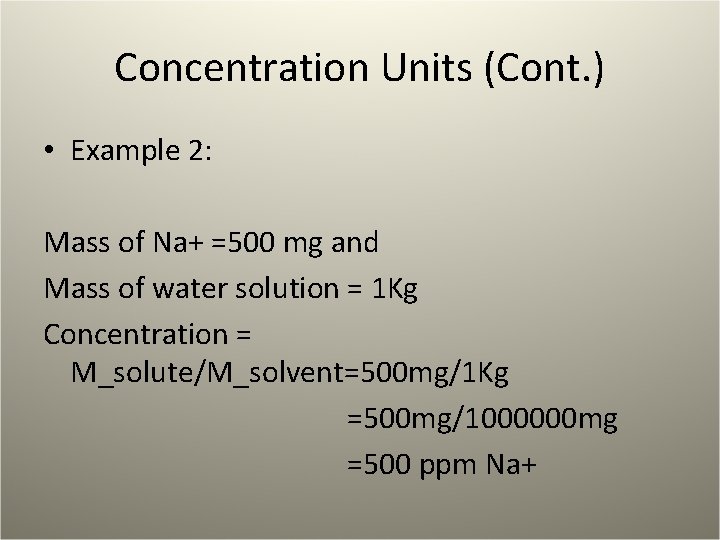
Concentration Units (Cont. ) • Example 2: Mass of Na+ =500 mg and Mass of water solution = 1 Kg Concentration = M_solute/M_solvent=500 mg/1 Kg =500 mg/1000000 mg =500 ppm Na+

Chemical Reactions In Groundwater • • • Ionic dissolution Carbonation Oxidation Reduction Hydrolysis Adsorption/Ion Exchange

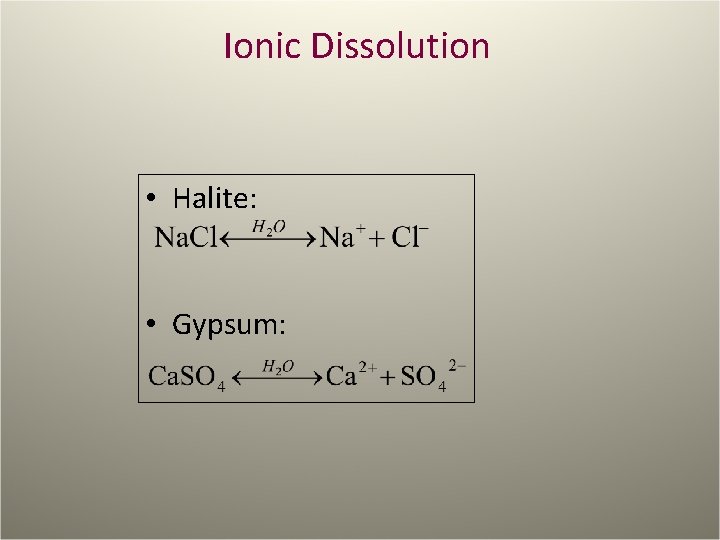
Ionic Dissolution • Halite: • Gypsum:

Solubility of Ionic Compounds Na. Cl (s) H 2 O Na + (aq) + Cl - (aq) The Dissolution of an Ionic Compound Cl - Ion Na+ Ion During this process, the oppositely charged ions separate from each other, become surrounded by water molecules and spread randomly throughout the solution 22

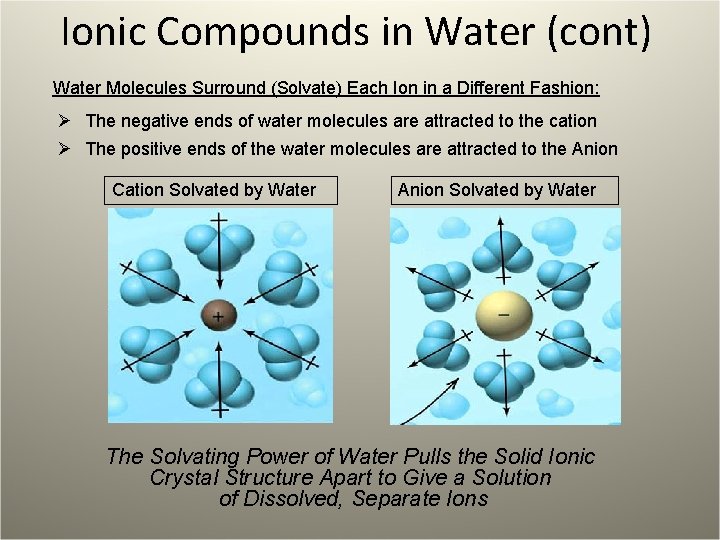
Ionic Compounds in Water (cont) Water Molecules Surround (Solvate) Each Ion in a Different Fashion: Ø The negative ends of water molecules are attracted to the cation Ø The positive ends of the water molecules are attracted to the Anion Cation Solvated by Water Anion Solvated by Water The Solvating Power of Water Pulls the Solid Ionic Crystal Structure Apart to Give a Solution of Dissolved, Separate Ions

Carbonation

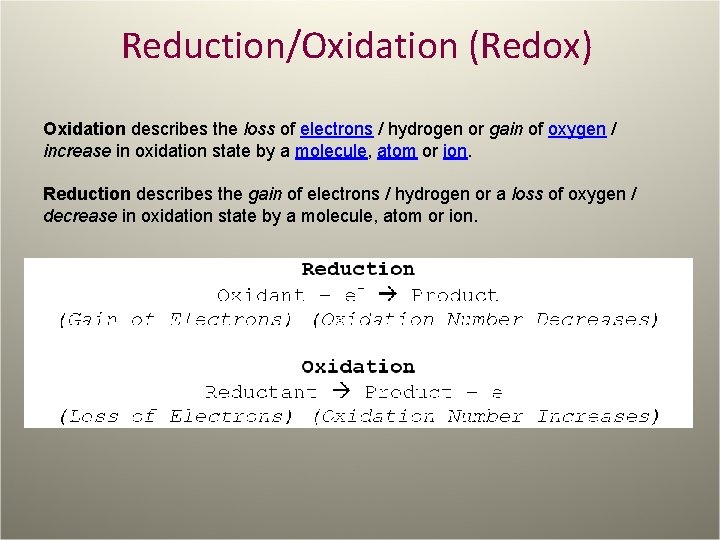
Reduction/Oxidation (Redox) Oxidation describes the loss of electrons / hydrogen or gain of oxygen / increase in oxidation state by a molecule, atom or ion. Reduction describes the gain of electrons / hydrogen or a loss of oxygen / decrease in oxidation state by a molecule, atom or ion.

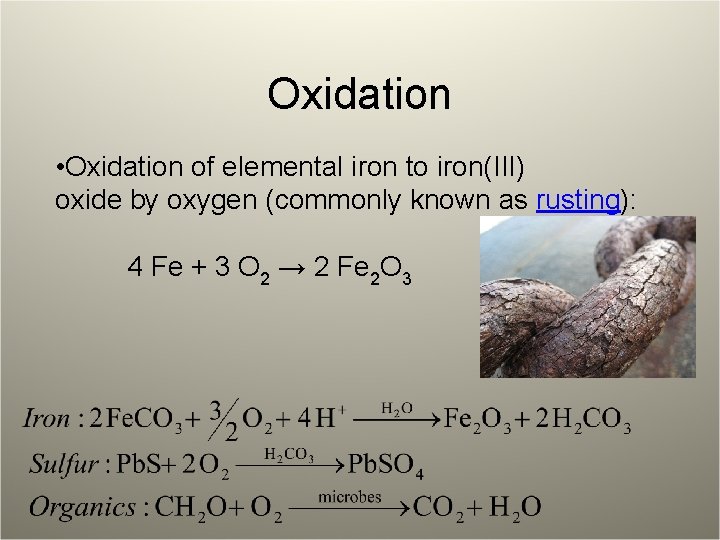
Oxidation • Oxidation of elemental iron to iron(III) oxide by oxygen (commonly known as rusting): 4 Fe + 3 O 2 → 2 Fe 2 O 3

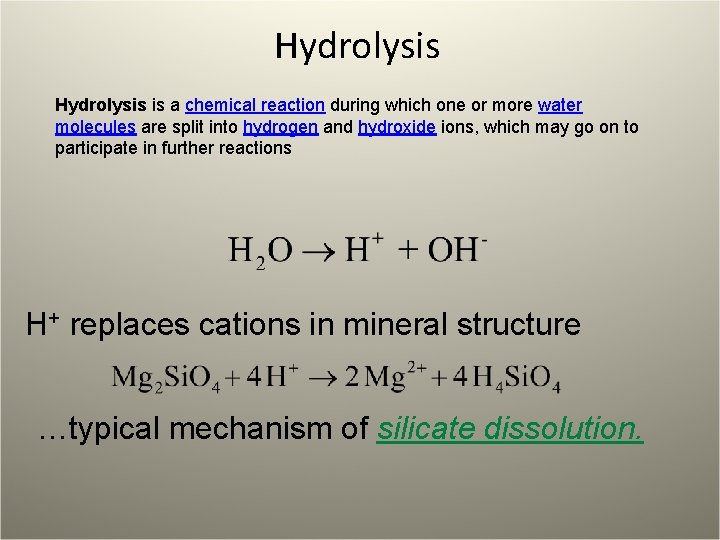
Hydrolysis is a chemical reaction during which one or more water molecules are split into hydrogen and hydroxide ions, which may go on to participate in further reactions H+ replaces cations in mineral structure …typical mechanism of silicate dissolution.

Ion Exchange Ion exchange is an exchange of ions between two electrolytes or between an electrolyte solution and a complex. In most cases the term is used to denote the processes of purification, separation, and decontamination of aqueous and other ion-containing solutions with solid polymeric or mineralic 'ion exchangers'. An electrolyte is any substance containing free ions that behaves as an electrically conductive medium Typical examples of ions that can bind to ion exchangers are: H+ (proton) and OH− (hydroxide) Single charged monoatomic ions like Na+, K+, or Cl− Double charged monoatomic ions like Ca 2+ or Mg 2+ Polyatomic inorganic ions like SO 42− or PO 43−

Acid Sources in GW Main acid sources: • carbonic acid (H 2 CO 3 from CO 2 dissociation in natural waters) • organic acids (from CO 2 respiration in soils) • sulfuric acid (volcanic, anthropogenic H 2 S)