Applied Geochemistry Lab Ch 6 Solubility Diagram Part
Applied Geochemistry & Lab Ch. 6 Solubility Diagram Part 1 JYU

1. Definitions q Solubility: the property of a solid, liquid, or gaseous substance called solute to dissolve in another solid, liquid, or gaseous substance called solvent to form a solution. This chapter specifically deals with solid solute in water to form aqueous solutions. The solubility also often means the extent of dissoslution. q Types of dissolution Congruent dissolution: the composition of the dissolved solid matches that of solutes produced Incongruent dissolution: doesn’t match
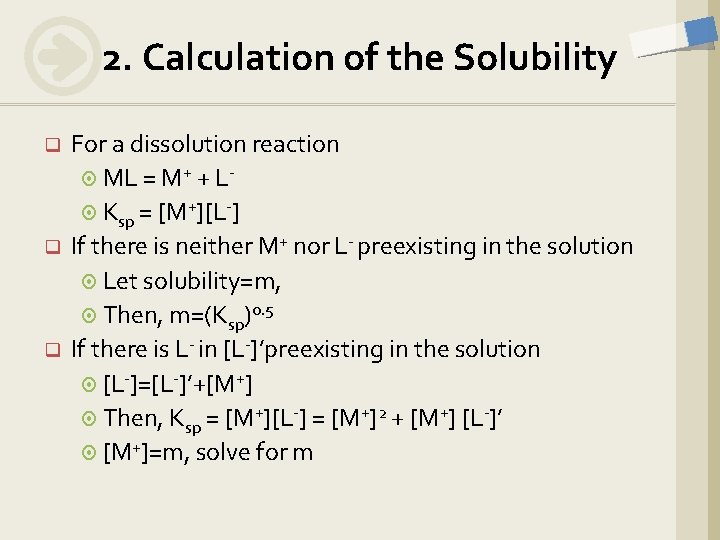
2. Calculation of the Solubility q q q For a dissolution reaction ML = M+ + L Ksp = [M+][L-] If there is neither M+ nor L- preexisting in the solution Let solubility=m, Then, m=(Ksp)0. 5 If there is L- in [L-]’preexisting in the solution [L-]=[L-]’+[M+] Then, Ksp = [M+][L-] = [M+]2 + [M+] [L-]’ [M+]=m, solve for m

3. Saturation q Precipitation vs. dissolution q Undersaturation dissolution Oversaturation (supersaturation) precipitation Saturation “equilibrium” Expression of the saturation Saturation ratio (SR) = IAP/Ksp Saturation index (SI) = log(SR) SR<1: US SR>1: OS SR=1: EQ SI<0: US SI>0: OS SI=0: EQ Affinity (A) = -RT*SI A>0: US A<0: OS A=0: EQ
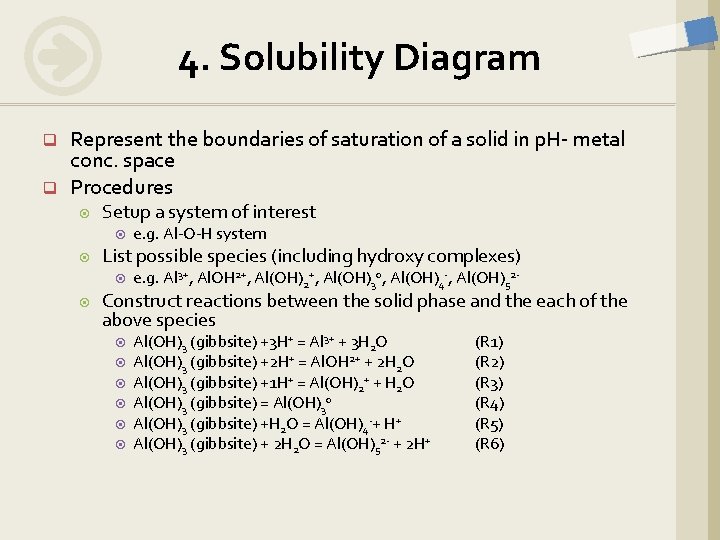
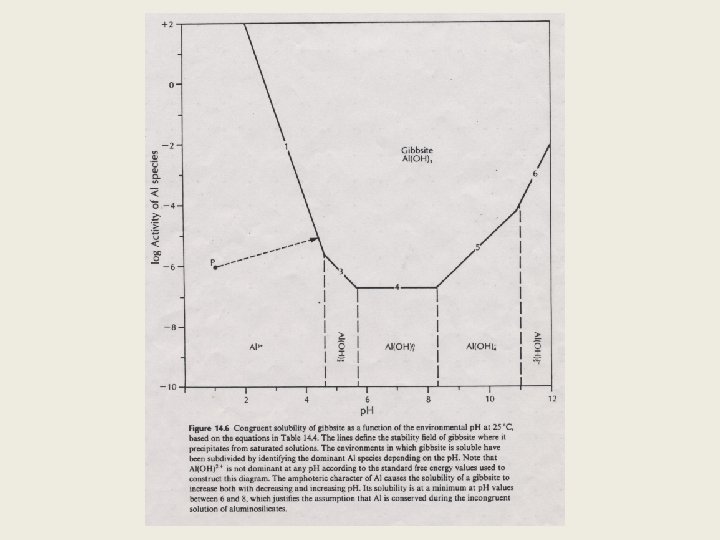
4. Solubility Diagram q q Represent the boundaries of saturation of a solid in p. H- metal conc. space Procedures Setup a system of interest List possible species (including hydroxy complexes) e. g. Al-O-H system e. g. Al 3+, Al. OH 2+, Al(OH)30, Al(OH)4 -, Al(OH)52 - Construct reactions between the solid phase and the each of the above species Al(OH)3 (gibbsite) +3 H+ = Al 3+ + 3 H 2 O Al(OH)3 (gibbsite) +2 H+ = Al. OH 2+ + 2 H 2 O Al(OH)3 (gibbsite) +1 H+ = Al(OH)2+ + H 2 O Al(OH)3 (gibbsite) = Al(OH)30 Al(OH)3 (gibbsite) +H 2 O = Al(OH)4 -+ H+ Al(OH)3 (gibbsite) + 2 H 2 O = Al(OH)52 - + 2 H+ (R 1) (R 2) (R 3) (R 4) (R 5) (R 6)
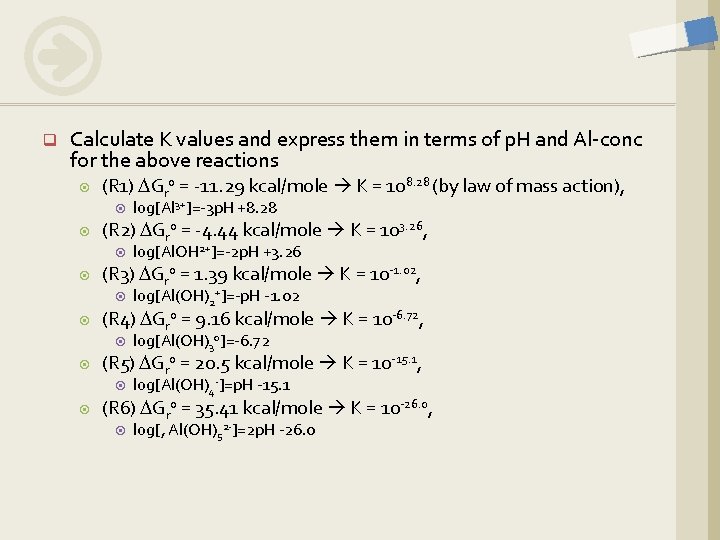
q Calculate K values and express them in terms of p. H and Al-conc for the above reactions (R 1) DGro = -11. 29 kcal/mole K = 108. 28 (by law of mass action), (R 2) DGro = -4. 44 kcal/mole K = 103. 26, log[Al(OH)30]=-6. 72 (R 5) DGro = 20. 5 kcal/mole K = 10 -15. 1, log[Al(OH)2+]=-p. H -1. 02 (R 4) DGro = 9. 16 kcal/mole K = 10 -6. 72, log[Al. OH 2+]=-2 p. H +3. 26 (R 3) DGro = 1. 39 kcal/mole K = 10 -1. 02, log[Al 3+]=-3 p. H +8. 28 log[Al(OH)4 -]=p. H -15. 1 (R 6) DGro = 35. 41 kcal/mole K = 10 -26. 0, log[, Al(OH)52 -]=2 p. H -26. 0
- Slides: 7