Applications of Molecular Rotational Spectroscopy for Chiral Analysis


- Slides: 14

Applications of Molecular Rotational Spectroscopy for Chiral Analysis Rotational Spectrum and Carbon Atom Structure of Dihydroartemisinic Acid Luca Evangelistsi, Lorenzo Spada, Nathan Seifert, and Brooks H. Pate Department of Chemistry, University of Virginia Justin Neill and Matt Muckle Bright. Spec, Inc. Hughes-Wilson Stark Modulation Spectrometer Phys. Rev. 71, 562 (1947) Commercialized by Hewlett-Packard in 1971 Frank Gupton Virginia Commonwealth University

Rotational Spectroscopy in Analytical Chemistry Can’t compete with “gold standard” methods that use mass spectrometry/ion detection: GC/MS (HPLC methods) 1) Reduction in the cost of consumables and technical expertise required 2) Samples that can’t use chromatography (reactive) – direct mixture analysis 3) Monitoring applications requiring “real-time” quantification (~1 min) 4) Analysis of isomers Isotopologues: Site-specific stable isotope analysis Chiral Analysis: Diastereomers and Enantiomers Isomer ratios are required Major Strength: Potential for Library-Free Analysis (Molecule identification by theory alone)

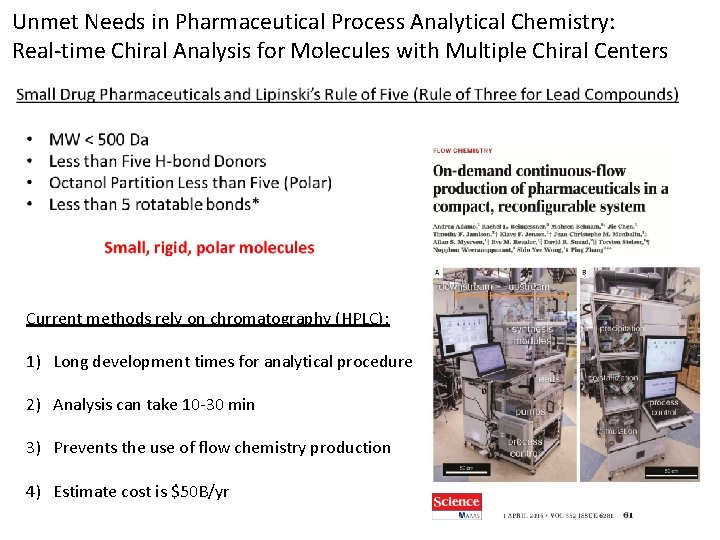
Unmet Needs in Analytical Chemistry: Routine Analysis of Molecules with Multiple Chiral Centers Many traditional pharmaceutical compounds exhibit multiple chiral centers, requiring methods that can at least separate the potential enantiomers and diastereomers from the API. Even more desirable is a method that can separate each of the potential isomeric impurities for accurate quantitation; however, this is rarely accomplished. https: //www. sigmaaldrich. com/content/dam/sigma-aldrich/docs/Supelco/Posters/1/T 413140 H_HPLC_Chiral_Multiple_Centers. pdf

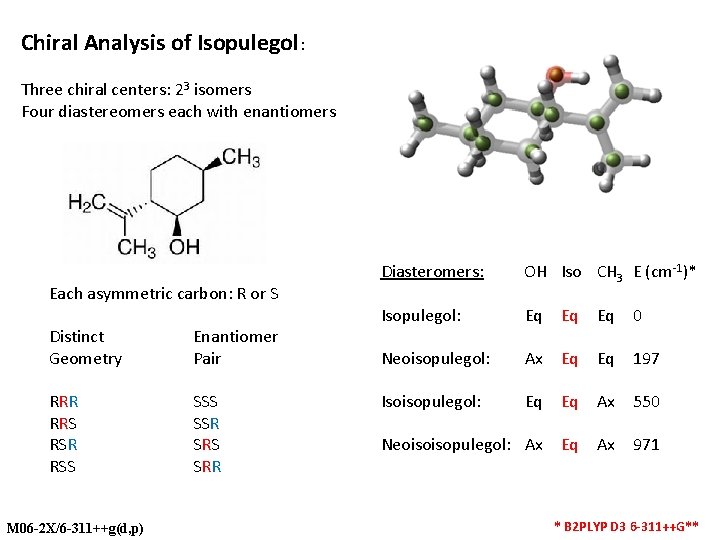
Chiral Analysis of Isopulegol: Three chiral centers: 23 isomers Four diastereomers each with enantiomers Each asymmetric carbon: R or S Distinct Geometry Enantiomer Pair RRR RRS RSR RSS SSR SRS SRR M 06 -2 X/6 -311++g(d, p) Diasteromers: OH Iso CH 3 E (cm-1)* Isopulegol: Eq Eq Eq 0 Neoisopulegol: Ax Eq Eq 197 Isoisopulegol: Eq Eq Ax 550 Neoisoisopulegol: Ax Eq Ax 971 * B 2 PLYP D 3 6 -311++G**

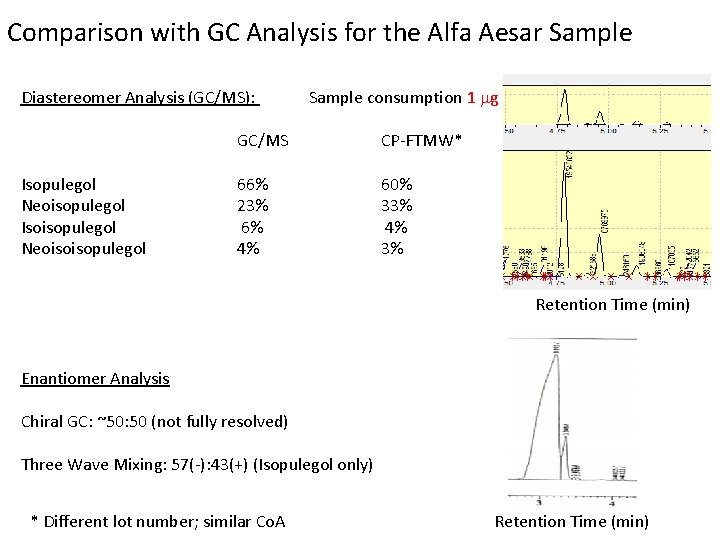
Comparison with GC Analysis for the Alfa Aesar Sample Diastereomer Analysis (GC/MS): Isopulegol Neoisopulegol Isoisopulegol Neoisoisopulegol Sample consumption 1 mg GC/MS CP-FTMW* 66% 23% 6% 4% 60% 33% 4% 3% Retention Time (min) Enantiomer Analysis Chiral GC: ~50: 50 (not fully resolved) Three Wave Mixing: 57(-): 43(+) (Isopulegol only) * Different lot number; similar Co. A Retention Time (min)

Unmet Needs in Pharmaceutical Process Analytical Chemistry: Real-time Chiral Analysis for Molecules with Multiple Chiral Centers Current methods rely on chromatography (HPLC): 1) Long development times for analytical procedure 2) Analysis can take 10 -30 min 3) Prevents the use of flow chemistry production 4) Estimate cost is $50 B/yr

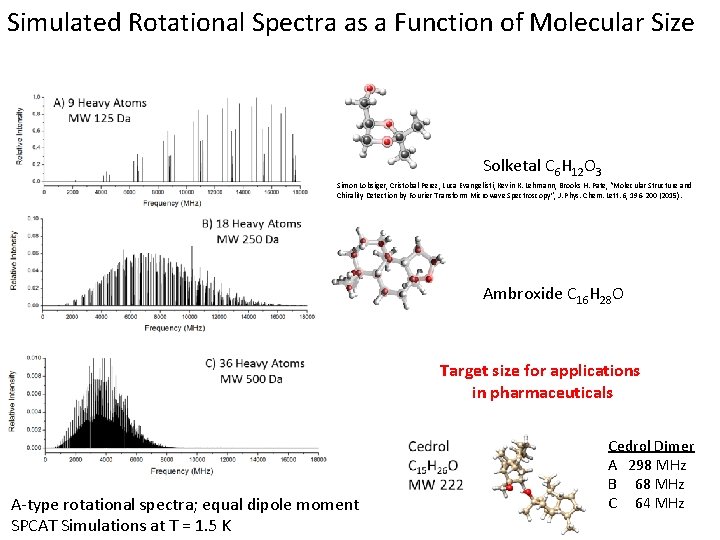
Simulated Rotational Spectra as a Function of Molecular Size Solketal C 6 H 12 O 3 Simon Lobsiger, Cristobal Perez, Luca Evangelisti, Kevin K. Lehmann, Brooks H. Pate, “Molecular Structure and Chirality Detection by Fourier Transform Microwave Spectroscopy”, J. Phys. Chem. Lett. 6, 196 -200 (2015). Ambroxide C 16 H 28 O Target size for applications in pharmaceuticals A-type rotational spectra; equal dipole moment SPCAT Simulations at T = 1. 5 K Cedrol Dimer A 298 MHz B 68 MHz C 64 MHz

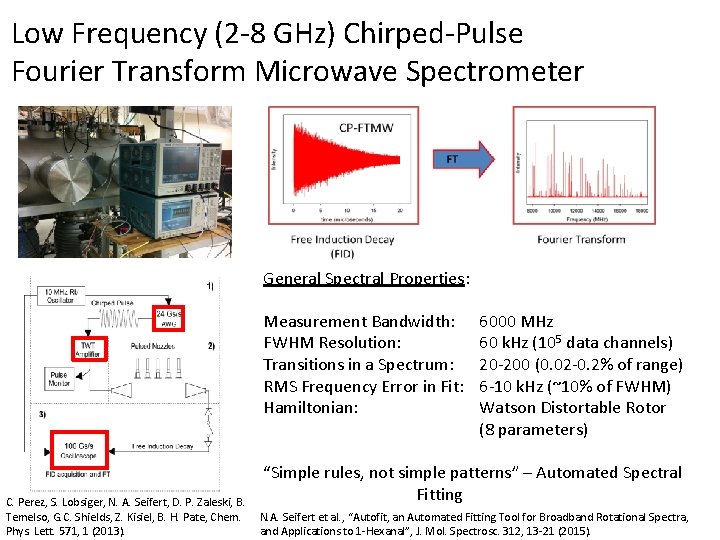
Low Frequency (2 -8 GHz) Chirped-Pulse Fourier Transform Microwave Spectrometer General Spectral Properties: Measurement Bandwidth: FWHM Resolution: Transitions in a Spectrum: RMS Frequency Error in Fit: Hamiltonian: C. Perez, S. Lobsiger, N. A. Seifert, D. P. Zaleski, B. Temelso, G. C. Shields, Z. Kisiel, B. H. Pate, Chem. Phys. Lett. 571, 1 (2013). 6000 MHz 60 k. Hz (105 data channels) 20 -200 (0. 02 -0. 2% of range) 6 -10 k. Hz (~10% of FWHM) Watson Distortable Rotor (8 parameters) “Simple rules, not simple patterns” – Automated Spectral Fitting N. A. Seifert et al. , “Autofit, an Automated Fitting Tool for Broadband Rotational Spectra, and Applications to 1 -Hexanal”, J. Mol. Spectrosc. 312, 13 -21 (2015).

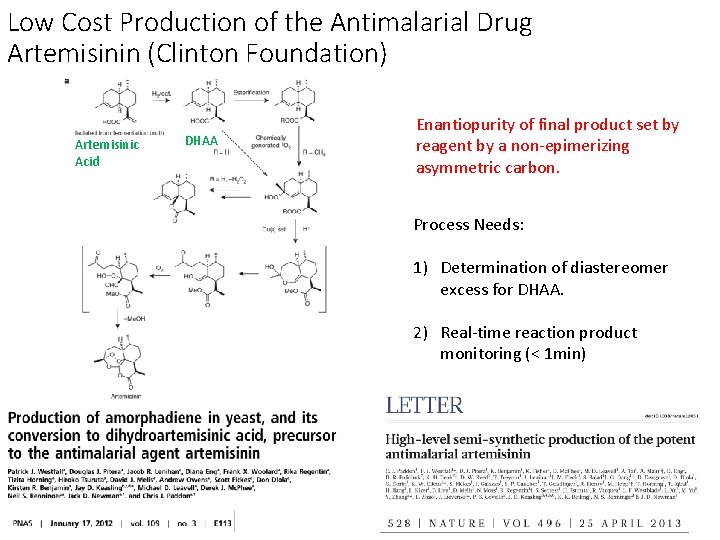
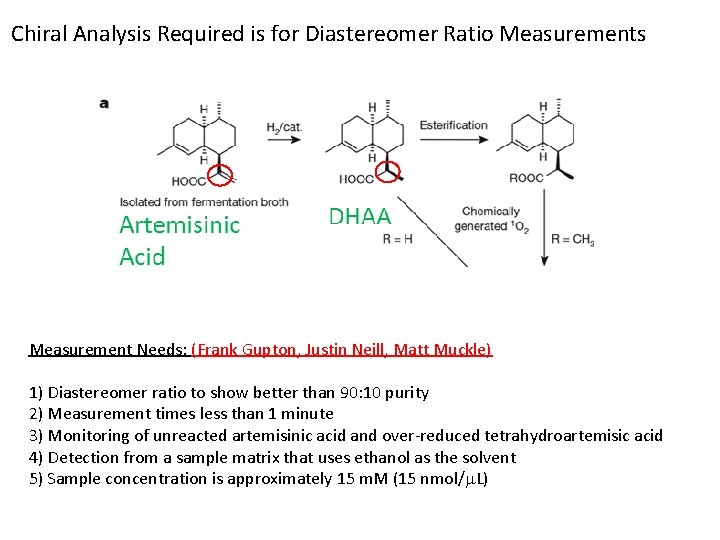
Low Cost Production of the Antimalarial Drug Artemisinin (Clinton Foundation) Artemisinic Acid DHAA Enantiopurity of final product set by reagent by a non-epimerizing asymmetric carbon. Process Needs: 1) Determination of diastereomer excess for DHAA. 2) Real-time reaction product monitoring (< 1 min)

Chiral Analysis Required is for Diastereomer Ratio Measurements Measurement Needs: (Frank Gupton, Justin Neill, Matt Muckle) 1) Diastereomer ratio to show better than 90: 10 purity 2) Measurement times less than 1 minute 3) Monitoring of unreacted artemisinic acid and over-reduced tetrahydroartemisic acid 4) Detection from a sample matrix that uses ethanol as the solvent 5) Sample concentration is approximately 15 m. M (15 nmol/m. L)

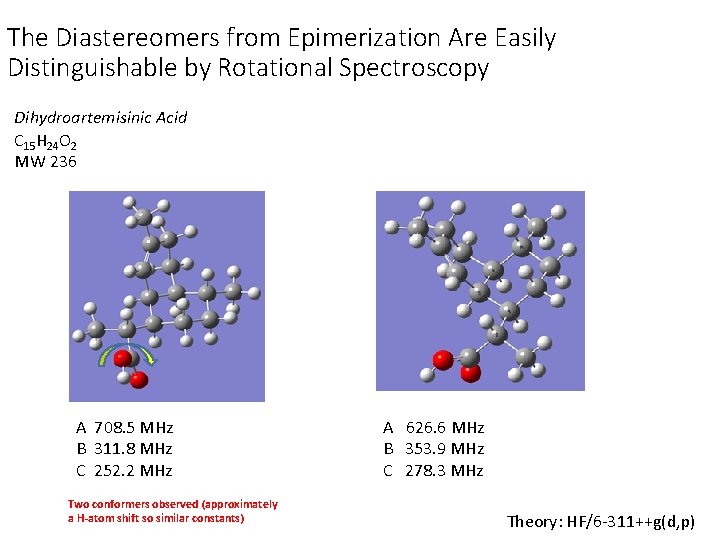
The Diastereomers from Epimerization Are Easily Distinguishable by Rotational Spectroscopy Dihydroartemisinic Acid C 15 H 24 O 2 MW 236 A 708. 5 MHz B 311. 8 MHz C 252. 2 MHz Two conformers observed (approximately a H-atom shift so similar constants) A 626. 6 MHz B 353. 9 MHz C 278. 3 MHz Theory: HF/6 -311++g(d, p)

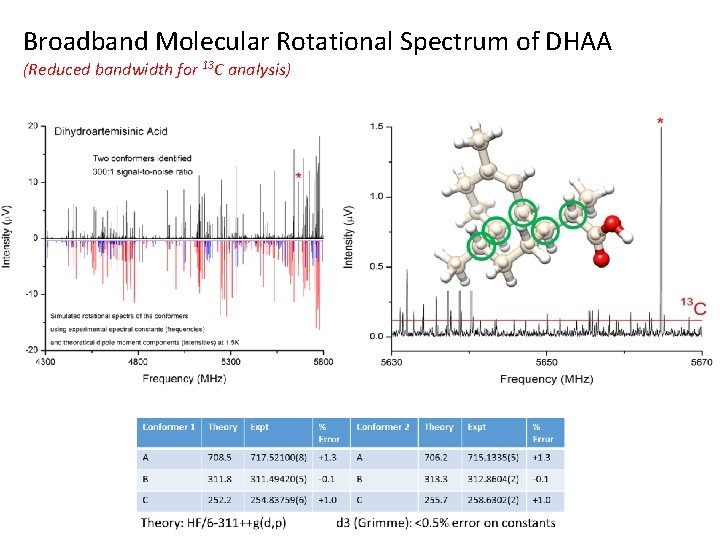
Broadband Molecular Rotational Spectrum of DHAA (Reduced bandwidth for 13 C analysis)

Acknowledgements National Science Foundation (Chemistry, CCI, MRI, I-Corps) National Radio Astronomy Observatory VA NC Alliance LSAMP University of Virginia Biosciences Health Research Corporation Bright. Spec, Inc. David Pratt, Steve Shipman, Bob Field, David Perry, Tom Gallagher Mike Mc. Carthy, Tony Remijan, Joanna Corby, Phil Jewel, Susanna Widicus-Weaver Rick Suenram, Frank Lovas, David Plusquellic Zbyszek Kisiel, George Shields, Berhane Temelso, Jeremy Richardson, Stuart Althorpe, David Wales, Alberto Lesarri, Sean Peebles, Rebecca Peebles, Gamil Guirgis, Jim Durig, Isabelle Kleiner, Bob Mc. Kellar, Kevin Lehmann Frank Gupton Pate Broadband Rotational Spectroscopy Group Gordon Brown, Kevin Douglass, Brian Dian, Steve Shipman Matt Muckle, Justin Neill, Dan Zaleski, Brent Harris, Amanda Steber, Nathan Seifert, Cristobal Perez, Simon Lobsiger, Luca Evangelisti, Lorenzo Spada, Jonathan Warren

Is Rotational Spectroscopy up for the Challenge? Quantitative chiral analysis of molecules with multiple chiral centers performed directly on reaction samples. • Can we identify molecules unambiguously at 500 Da? Can we develop efficient conformational analysis methods Distinguishability of conformers and diastereomers Accuracy of quantum chemistry methods Ability to resolve isotopologues • Can we achieve sensitivity for high purity measurements (>99. 5%)? Sampling methods for large molecules Spectral clutter at 1000: 1 especially in complex sample matrices Instruments for low-frequency rotational spectroscopy Potential for cavity-enhanced instrument for rapid monitoring • Can we meet the challenges of a single instrument for complete chiral analysis? Diastereomer and enantiomer analysis Enantiomer analysis in the high enantiopurity limit (ee > 99. 5) Three wave mixing or chiral tagging