Applications of ETSNOCV method in description of various

![Quadruple bond; Re 2 Cl 82 [-84. 3 kcal/mol] 2[-65. 5 kcal/mol] 1[-65. 5 Quadruple bond; Re 2 Cl 82 [-84. 3 kcal/mol] 2[-65. 5 kcal/mol] 1[-65. 5](https://slidetodoc.com/presentation_image_h2/5004989d08252310f1e87dd9a4540890/image-14.jpg)


- Slides: 27

Applications of ETS-NOCV method in description of various types of chemical bonds Mariusz P. Mitoraj Jagiellonian University Cracow, Poland Department of Theoretical Chemistry ADF webinar, Kraków-rest-of-the-world, 28 th Feb. , 2014

Examples of theoretical quantities for visualization of chemical bond ADF webinar, Kraków-restoftheworld, 28 th Feb. , 2014

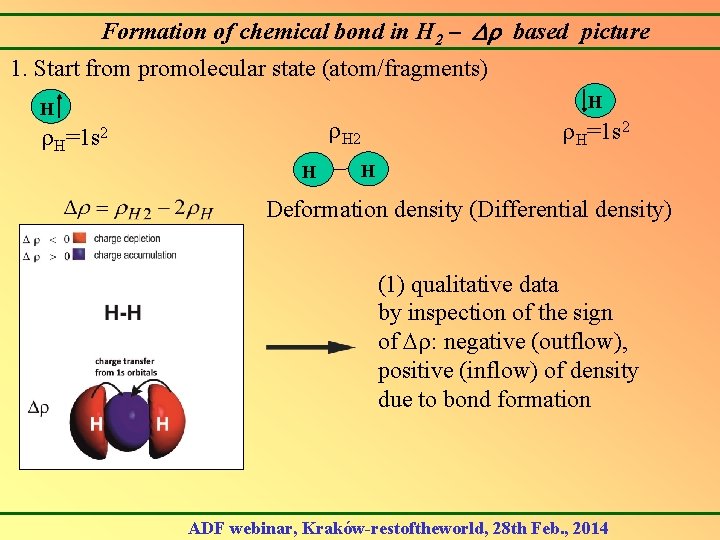
Formation of chemical bond in H 2 – based picture 1. Start from promolecular state (atom/fragments) H H H 2 H=1 s 2 H Deformation density (Differential density) (1) qualitative data by inspection of the sign of : negative (outflow), positive (inflow) of density due to bond formation ADF webinar, Kraków-restoftheworld, 28 th Feb. , 2014

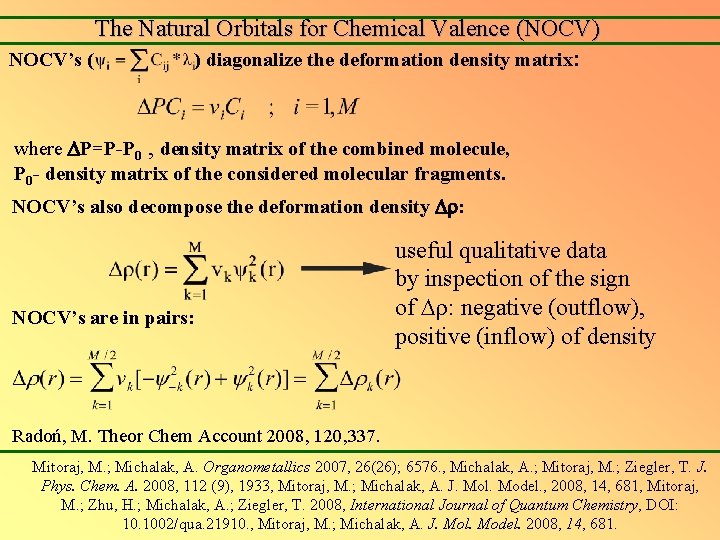
The Natural Orbitals for Chemical Valence (NOCV) NOCV’s ( ) diagonalize the deformation density matrix: where P=P-P 0 , density matrix of the combined molecule, P 0 - density matrix of the considered molecular fragments. NOCV’s also decompose the deformation density : NOCV’s are in pairs: useful qualitative data by inspection of the sign of : negative (outflow), positive (inflow) of density Radoń, M. Theor Chem Account 2008, 120, 337. Mitoraj, M. ; Michalak, A. Organometallics 2007, 26(26); 6576. , Michalak, A. ; Mitoraj, M. ; Ziegler, T. J. Phys. Chem. A. 2008, 112 (9), 1933, Mitoraj, M. ; Michalak, A. J. Mol. Model. , 2008, 14, 681, Mitoraj, M. ; Zhu, H. ; Michalak, A. ; Ziegler, T. 2008, International Journal of Quantum Chemistry, DOI: 10. 1002/qua. 21910. , Mitoraj, M. ; Michalak, A. J. Mol. Model. 2008, 14, 681.

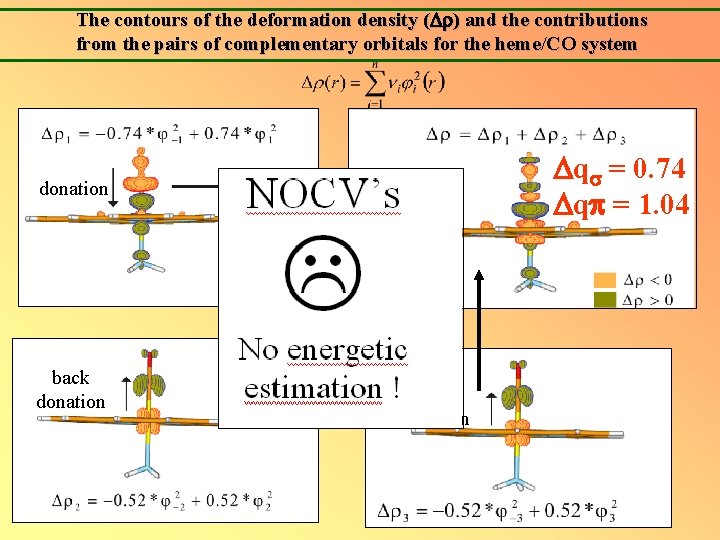
The contours of the deformation density ( ) and the contributions from the pairs of complementary orbitals for the heme/CO system q = 0. 74 q = 1. 04 donation back donation

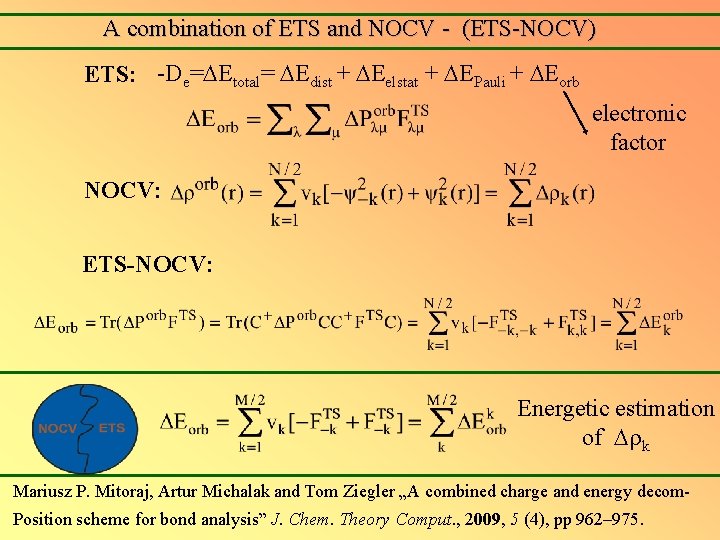
A combination of ETS and NOCV - (ETS-NOCV) ETS: -De= Etotal= Edist + Eelstat + EPauli + Eorb electronic factor NOCV: ETS-NOCV: Energetic estimation of k Mariusz P. Mitoraj, Artur Michalak and Tom Ziegler „A combined charge and energy decom. Position scheme for bond analysis” J. Chem. Theory Comput. , 2009, 5 (4), pp 962– 975.

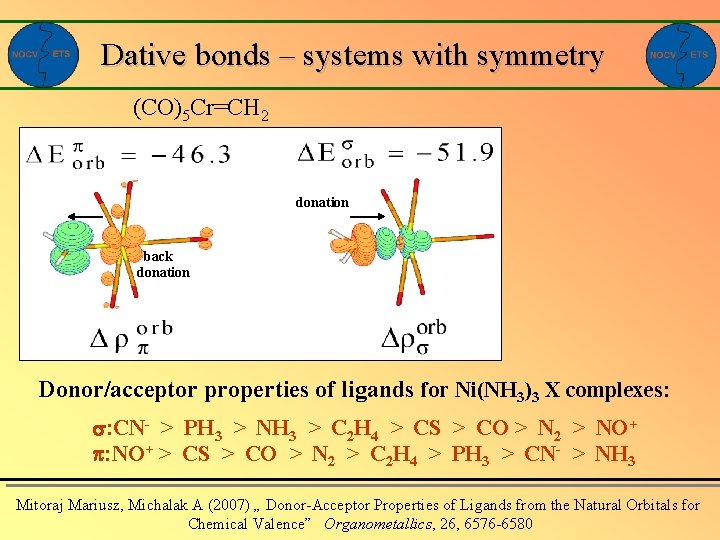
Dative bonds – systems with symmetry (CO)5 Cr=CH 2 donation back donation Donor/acceptor properties of ligands for Ni(NH 3)3 X complexes: : CN- > PH 3 > NH 3 > C 2 H 4 > CS > CO > N 2 > NO+ : NO+ > CS > CO > N 2 > C 2 H 4 > PH 3 > CN- > NH 3 Mitoraj Mariusz, Michalak A (2007) „ Donor-Acceptor Properties of Ligands from the Natural Orbitals for Chemical Valence” Organometallics, 26, 6576 -6580

Dative Bond NH 3 BH 3 - Calculations • Define closed shell fragments, NH 3 and BH 3 • Run SP calculations to get the fragment MO’s • Run SP ETS-NOCV calculations for whole molecule in the basis of previously calculated fragment MO’s

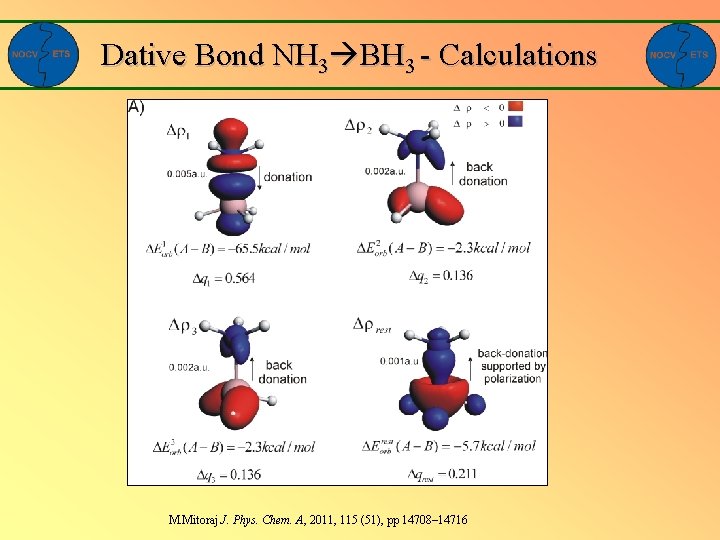
Dative Bond NH 3 BH 3 - Calculations M. Mitoraj J. Phys. Chem. A, 2011, 115 (51), pp 14708– 14716

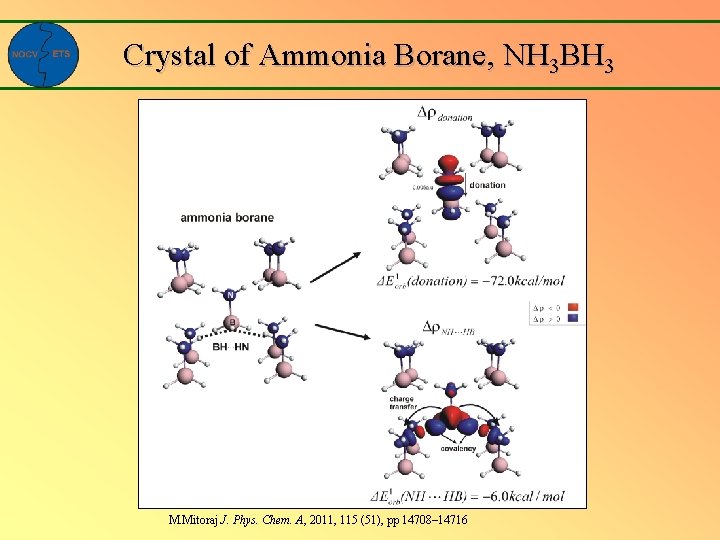
Crystal of Ammonia Borane, NH 3 BH 3 M. Mitoraj J. Phys. Chem. A, 2011, 115 (51), pp 14708– 14716

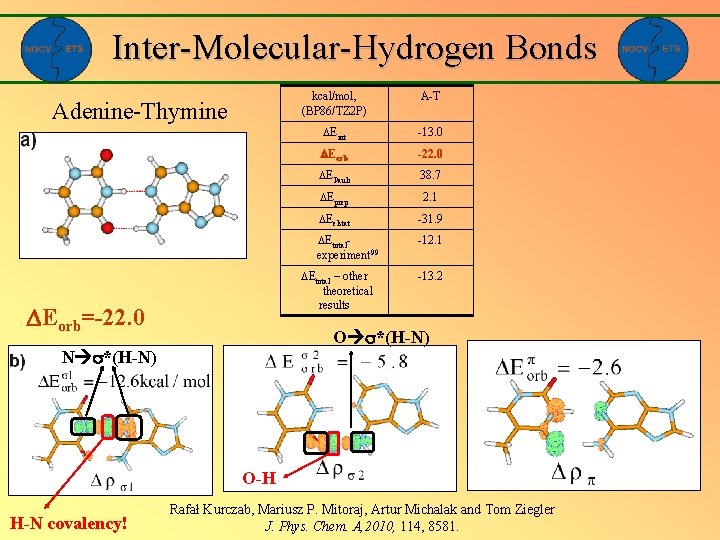
Inter-Molecular-Hydrogen Bonds Adenine-Thymine kcal/mol, (BP 86/TZ 2 P) A-T Eint -13. 0 Eorb -22. 0 EPauli 38. 7 Eprep 2. 1 Eelstat -31. 9 Etotalexperiment 99 -12. 1 Etotal – other theoretical results Eorb=-22. 0 -13. 2 O *(H-N) N *(H-N) O-H H-N covalency! Rafał Kurczab, Mariusz P. Mitoraj, Artur Michalak and Tom Ziegler J. Phys. Chem. A, 2010, 114, 8581.

Hydrogen Bond A-T Calculations • Define closed shell fragments, Adenine and Thymine • Run SP calculations to get the fragment MO’s • Run SP ETS-NOCV calculations for whole molecule in the basis of previously calculated fragment MO’s ADF webinar, Kraków-restoftheworld, 28 th Feb. , 2014

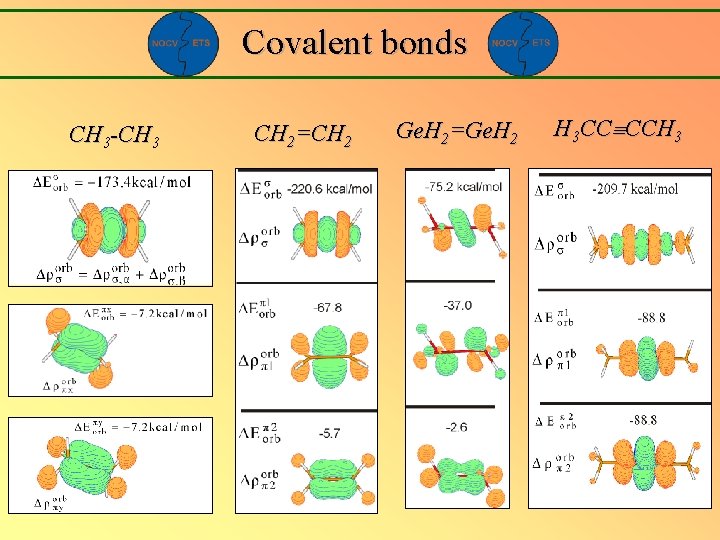
Covalent bonds CH 3 -CH 3 CH 2=CH 2 Ge. H 2=Ge. H 2 H 3 CC CCH 3
![Quadruple bond Re 2 Cl 82 84 3 kcalmol 265 5 kcalmol 165 5 Quadruple bond; Re 2 Cl 82 [-84. 3 kcal/mol] 2[-65. 5 kcal/mol] 1[-65. 5](https://slidetodoc.com/presentation_image_h2/5004989d08252310f1e87dd9a4540890/image-14.jpg)
Quadruple bond; Re 2 Cl 82 [-84. 3 kcal/mol] 2[-65. 5 kcal/mol] 1[-65. 5 kcal/mol] [-1. 3 kcal/mol]

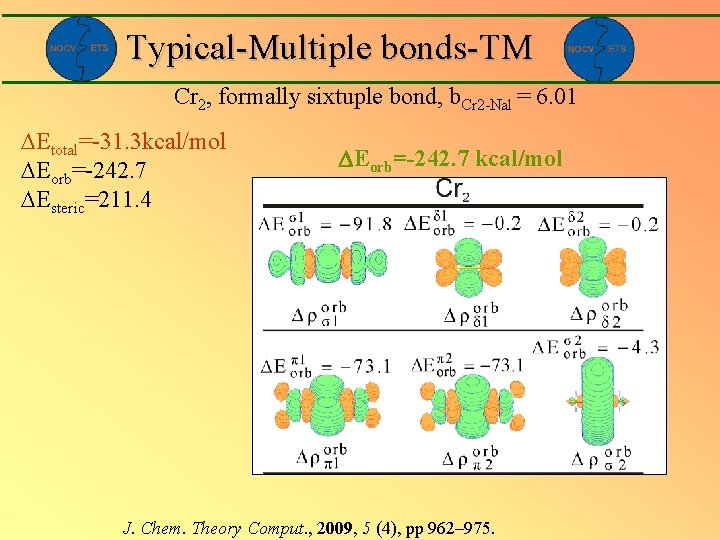
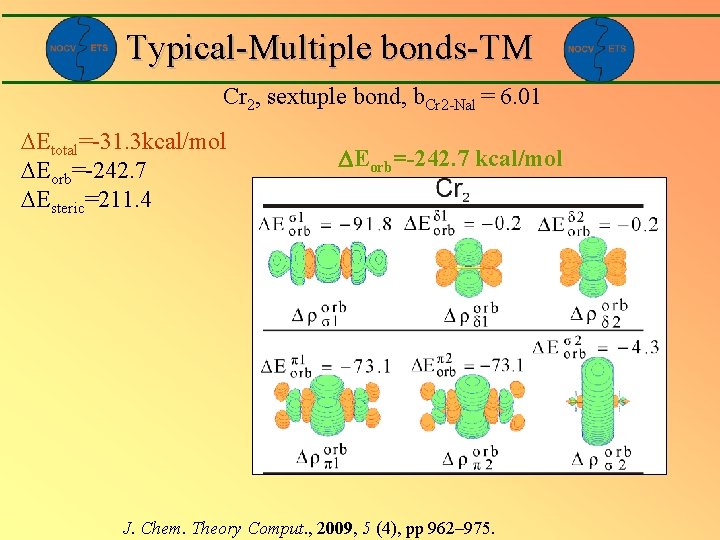
Typical-Multiple bonds-TM Cr 2, formally sixtuple bond, b. Cr 2 -Nal = 6. 01 Etotal=-31. 3 kcal/mol Eorb=-242. 7 Esteric=211. 4 Eorb=-242. 7 kcal/mol J. Chem. Theory Comput. , 2009, 5 (4), pp 962– 975.

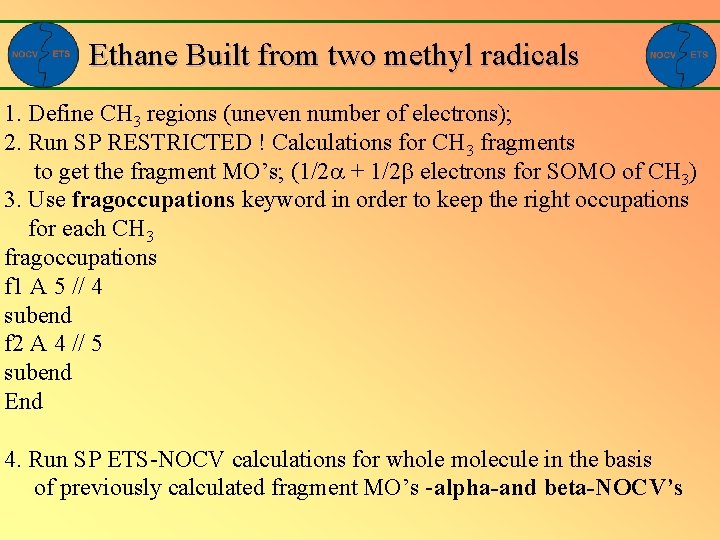
Ethane Built from two methyl radicals 1. Define CH 3 regions (uneven number of electrons); 2. Run SP RESTRICTED ! Calculations for CH 3 fragments to get the fragment MO’s; (1/2 + 1/2 electrons for SOMO of CH 3) 3. Use fragoccupations keyword in order to keep the right occupations for each CH 3 fragoccupations f 1 A 5 // 4 subend f 2 A 4 // 5 subend End 4. Run SP ETS-NOCV calculations for whole molecule in the basis of previously calculated fragment MO’s -alpha-and beta-NOCV’s

Typical-Multiple bonds-TM Cr 2, sextuple bond, b. Cr 2 -Nal = 6. 01 Etotal=-31. 3 kcal/mol Eorb=-242. 7 Esteric=211. 4 Eorb=-242. 7 kcal/mol J. Chem. Theory Comput. , 2009, 5 (4), pp 962– 975.

Cr 2 Built from two Cr + Cr 1. Define Cr regions (uneven number of electrons); 2. Run SP RESTRICTED ! Calculations for Cr fragments using OCCUPATION keyword (A 18 1 1 1); Should be like Cr but it is Cr 6* (1/2 + 1/2 ) so we must: 3. Use fragoccupations keyword in order to keep the right occupations for each Cr. fragoccupations Region_1 A 15//9 subend Region_2 A 9//15 subend End Cr 4. Run SP ETS-NOCV calculations for whole molecule in the basis of previously calculated fragment MO’s

Better Fragment MO’s from unrestriced run: advanced ’manual’ usage ADF uses in principle only ‚rectricted’ fragments, however, one might cheat him 1. Run open shell calculations for a given fragment; 2. Save tape 21 ascii (dmpkf) copy the final MO’s (from the section Eig-Core. SFO_A, MO’s expressed in SFO); 3. Run fragment calculations by setting SCF=0 wrong MO’s save tape 21 ascii find Eig-Core. SFO_A section, then: 4. Take MO’s and occupations, energies, from step 2 and paste them to Tape 21 from step 3…. . it gives you right fragment MO’s Transform ascii binary(udmpkf); 5. Rerun final ETSNOCV calculations (do not recalculate the fragments!)

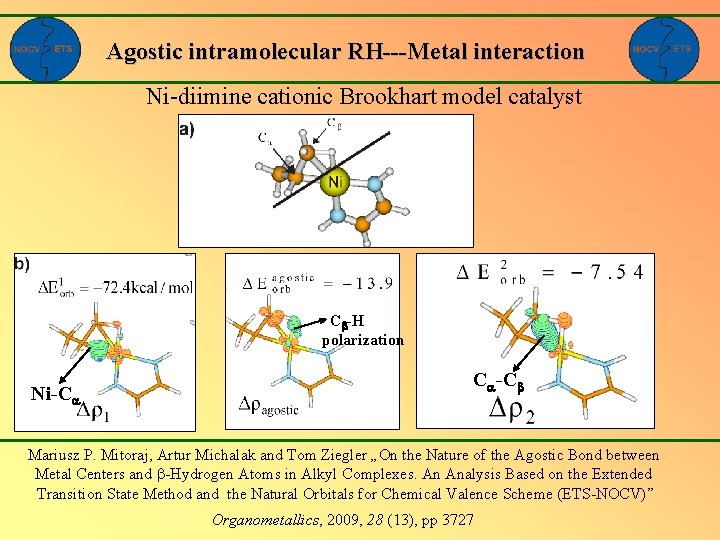
Agostic intramolecular RH---Metal interaction Ni-diimine cationic Brookhart model catalyst Cb-H polarization Ni-Ca Ca-Cb Mariusz P. Mitoraj, Artur Michalak and Tom Ziegler „On the Nature of the Agostic Bond between Metal Centers and -Hydrogen Atoms in Alkyl Complexes. An Analysis Based on the Extended Transition State Method and the Natural Orbitals for Chemical Valence Scheme (ETS-NOCV)” Organometallics, 2009, 28 (13), pp 3727

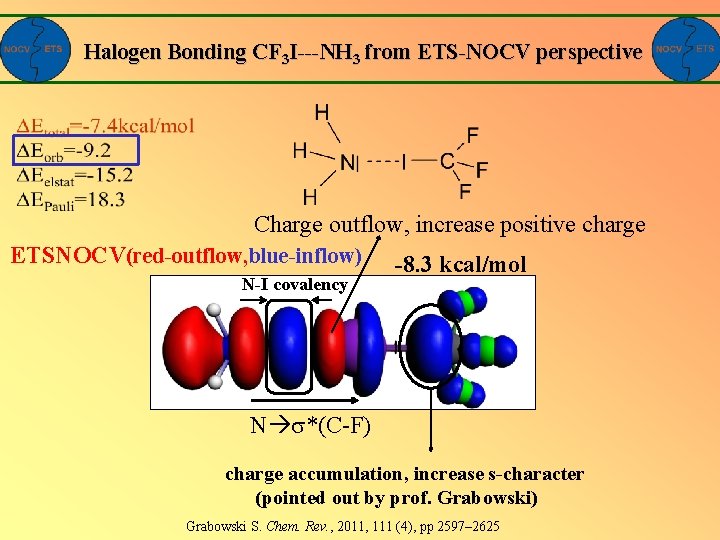
Halogen Bonding CF 3 I---NH 3 from ETS-NOCV perspective Charge outflow, increase positive charge ETSNOCV(red-outflow, blue-inflow) N-I covalency -8. 3 kcal/mol N *(C-F) charge accumulation, increase s-character (pointed out by prof. Grabowski) Grabowski S. Chem. Rev. , 2011, 111 (4), pp 2597– 2625

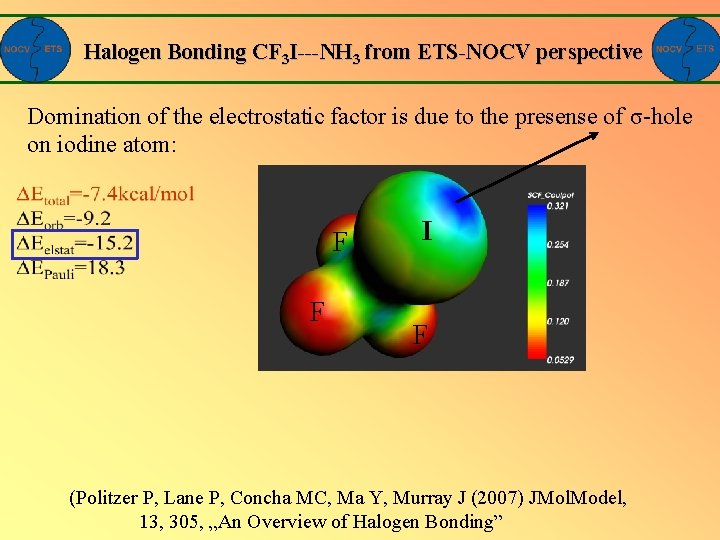
Halogen Bonding CF 3 I---NH 3 from ETS-NOCV perspective Domination of the electrostatic factor is due to the presense of σ-hole on iodine atom: F F I F (Politzer P, Lane P, Concha MC, Ma Y, Murray J (2007) JMol. Model, 13, 305, „An Overview of Halogen Bonding”

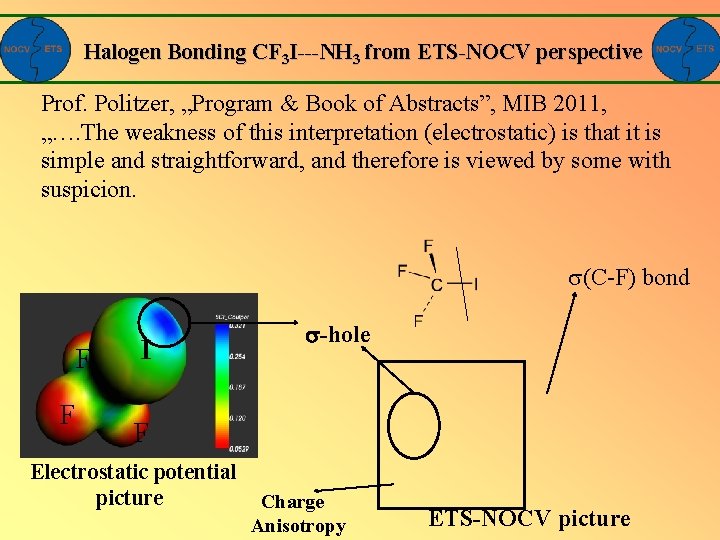
Halogen Bonding CF 3 I---NH 3 from ETS-NOCV perspective Prof. Politzer, „Program & Book of Abstracts”, MIB 2011, „…. The weakness of this interpretation (electrostatic) is that it is simple and straightforward, and therefore is viewed by some with suspicion. (C-F) bond F F I -hole F Electrostatic potential picture Charge Anisotropy ETS-NOCV picture

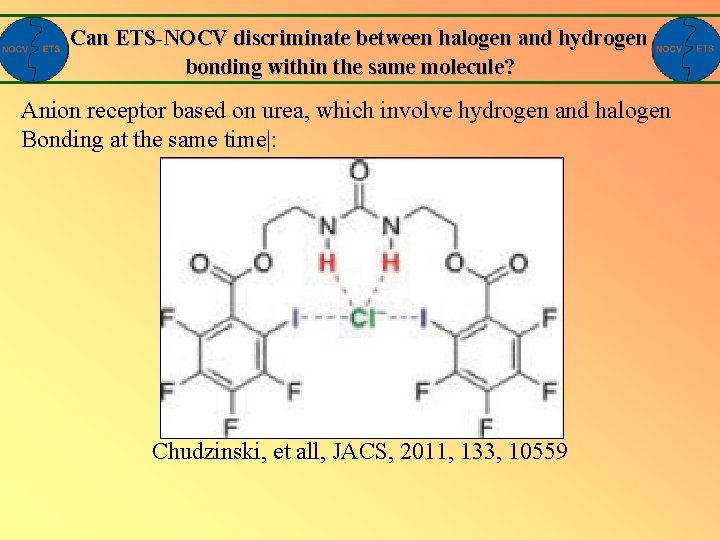
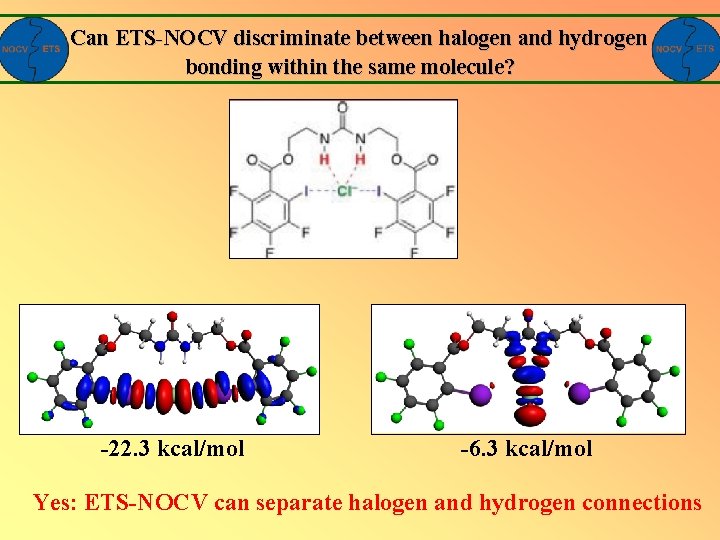
Can ETS-NOCV discriminate between halogen and hydrogen bonding within the same molecule? Anion receptor based on urea, which involve hydrogen and halogen Bonding at the same time|: Chudzinski, et all, JACS, 2011, 133, 10559

Can ETS-NOCV discriminate between halogen and hydrogen bonding within the same molecule? -22. 3 kcal/mol -6. 3 kcal/mol Yes: ETS-NOCV can separate halogen and hydrogen connections

Cl- / rest • Define closed shell fragments, Cl minus + rest of the complex • Run SP calculations to get the fragment MO’s • Run SP ETS-NOCV calculations for whole molecule in the basis of previously calculated fragment MO’s ADF webinar, Kraków-restoftheworld, 28 th Feb. , 2014

Thank You very much for Your Attention! ADF webinar, Kraków-restoftheworld, 28 th Feb. , 2014
 What is the purpose of symposium
What is the purpose of symposium Java applications begin execution at method
Java applications begin execution at method Double description
Double description Are elephants afraid of mice independent variable
Are elephants afraid of mice independent variable Website is a collection of html *
Website is a collection of html * Cyclical theory of social change
Cyclical theory of social change How to shrink a rubber band
How to shrink a rubber band Position of patient in bed
Position of patient in bed Acts done with fear examples
Acts done with fear examples The analysis of a literary text through various lenses
The analysis of a literary text through various lenses Castle clock analog computer
Castle clock analog computer Stages of development
Stages of development Various marketing concepts
Various marketing concepts State various types of track electrification system
State various types of track electrification system Distribution channels in business
Distribution channels in business How do consumers respond to various marketing efforts
How do consumers respond to various marketing efforts Simplified format of business letter
Simplified format of business letter Variety adj
Variety adj List various rapid prototyping data formats
List various rapid prototyping data formats Various modules of digital marketing
Various modules of digital marketing Characteristics of bad srs document
Characteristics of bad srs document Sahrd
Sahrd Various control structure in vb
Various control structure in vb Steps in the portfolio management process
Steps in the portfolio management process Media types
Media types Movement by day ncc
Movement by day ncc Function of management
Function of management Alphabetical classification of crude drugs
Alphabetical classification of crude drugs