Application of the colligative properties Determination of Molecular







- Slides: 10

Application of the colligative properties Determination of Molecular Masses (Session 22) Ciencias de la Tierra II

Colligative Properties • With the colligative properties of the solutions we can determine the molar masses or molecular of solutes dissolved in them. • This is an important application when we want to identify a unknown solute because from the molecular mass and percentage composition can determine the chemical formula of a solute. Ciencias de la Tierra II

• The colligative properties decrease od the freezing point and elevation of the boiling point are used to determine the molar or molecular masses with the methods know as: • Cryoscopy: decrease of the freezing point • Ebullioscopy: increase of the boiling point Ciencias de la Tierra II

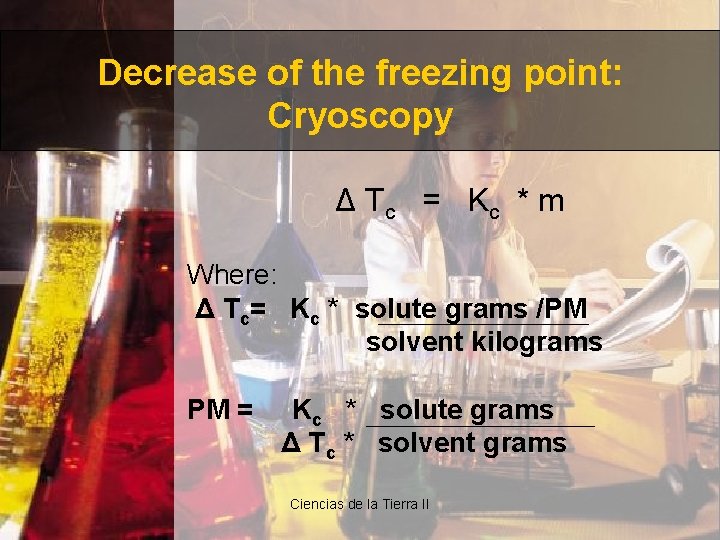
Decrease of the freezing point: Cryoscopy Δ Tc = Kc * m Where: Δ Tc= Kc * solute grams /PM solvent kilograms PM = Kc * solute grams Δ Tc * solvent grams Ciencias de la Tierra II

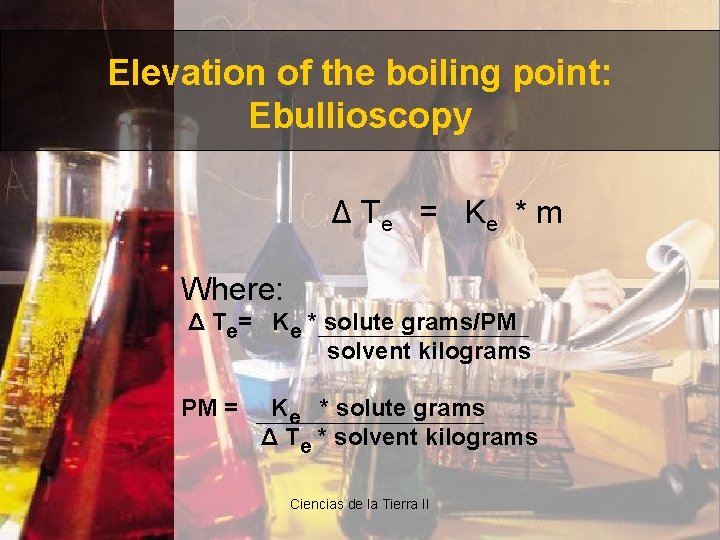
Elevation of the boiling point: Ebullioscopy Δ T e = Ke * m Where: Δ Te= Ke * solute grams/PM solvent kilograms PM = Ke * solute grams Δ Te * solvent kilograms Ciencias de la Tierra II

Problem #1 When in 150 g of water 0. 946 g of fructose (sugar of fruits) are dissolved, we can observe in the resulting solution has a freezing point of– 0. 0651 0 C. What is the molecular mass of the fructose? Ciencias de la Tierra II

Problem #2 A solution that contains 22. 0 g of ascorbic acid (vitamin C) in 100 g of water freezes at -2. 33 0 C. Which is molecular mass of ascorbic acid? Ciencias de la Tierra II

Problem #3 A solution has 22. 0 g ascorbic acid (vitamin C) in 100 g of water it freezes at -2. 33 0 C. What is the molecular mass of ascorbic acid? Ciencias de la Tierra II

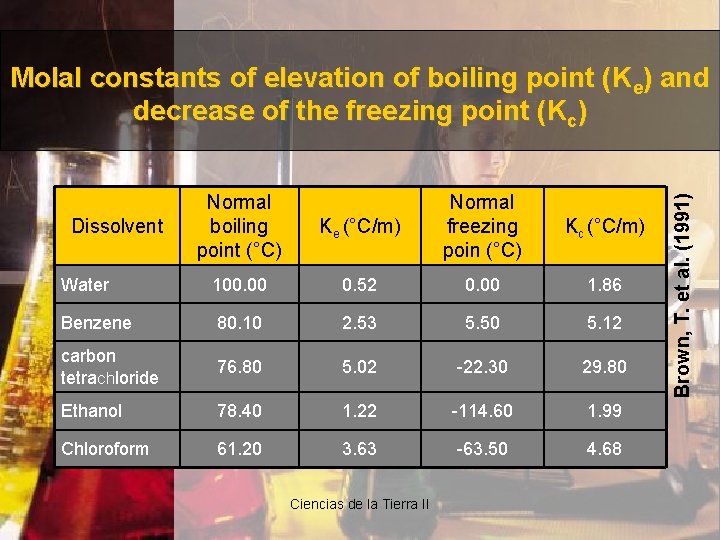
Normal boiling point (°C) Ke (°C/m) Normal freezing poin (°C) Kc (°C/m) Water 100. 00 0. 52 0. 00 1. 86 Benzene 80. 10 2. 53 5. 50 5. 12 carbon tetrachloride 76. 80 5. 02 -22. 30 29. 80 Ethanol 78. 40 1. 22 -114. 60 1. 99 Chloroform 61. 20 3. 63 -63. 50 4. 68 Dissolvent Ciencias de la Tierra II Brown, T. et al. (1991) Molal constants of elevation of boiling point (Ke) and decrease of the freezing point (Kc)

Bibliography • Brown, T. et al. (1991) Chemistry: The Central Science. 5 th ed. United States of America: Prentice Hall. • Burns, R. (1996) Chemistry Fundaments. 2 nd. ed. United States of America: Prentice Hall. • Chang, R. (1992). Chemistry. 4 th ed. United States of America: Mc. Graw Hill. Ciencias de la Tierra II
 Raoult's law physical pharmacy
Raoult's law physical pharmacy Dot
Dot Is molarity a colligative property
Is molarity a colligative property Colligative property definition
Colligative property definition Colligative properties definition
Colligative properties definition Solutions chemistry regents questions
Solutions chemistry regents questions Colligative properties of milk
Colligative properties of milk Non colligative properties
Non colligative properties Colligative properties worksheet
Colligative properties worksheet Colligative properties depend
Colligative properties depend Freezing point depression examples in real life
Freezing point depression examples in real life