APPLICATION OF PKPD MODELING IN DRUG DEVELOPMENT Amarnath




- Slides: 28

APPLICATION OF PK/PD MODELING IN DRUG DEVELOPMENT Amarnath Sharma, Ph. D. Pfizer Global R & D Groton, CT

Objectives of Early Drug Development u Identification of critical risk factors prior to investment in full clinical development Þ selection of better compounds u Provide critical data to identify safe and effective dose and dose regimens Þ more efficient development

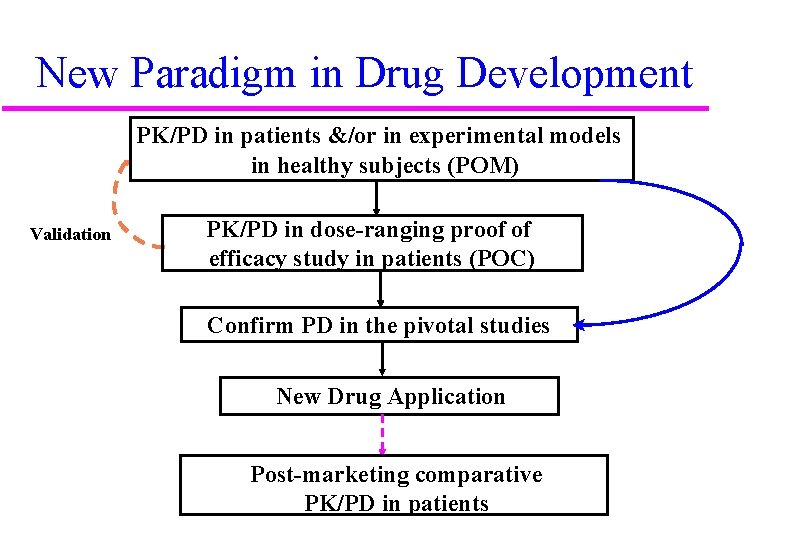
New Paradigm in Drug Development PK/PD in patients &/or in experimental models in healthy subjects (POM) Validation PK/PD in dose-ranging proof of efficacy study in patients (POC) Confirm PD in the pivotal studies New Drug Application Post-marketing comparative PK/PD in patients

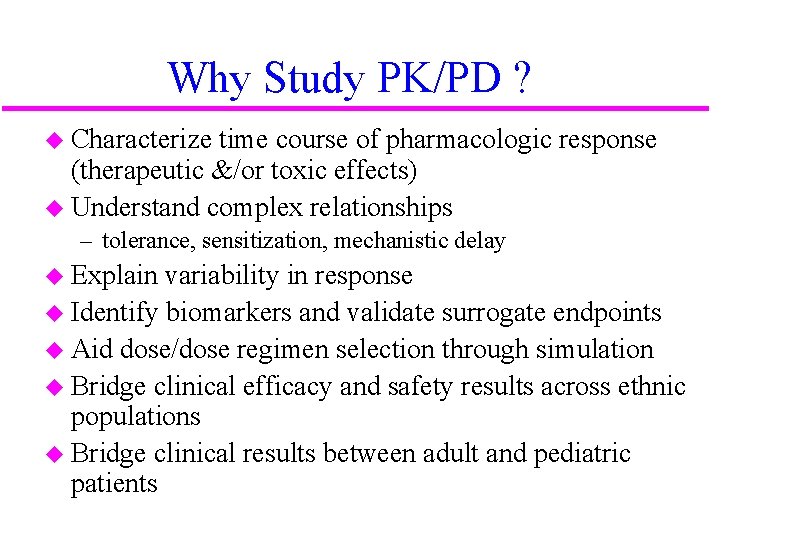
Why Study PK/PD ? u Characterize time course of pharmacologic response (therapeutic &/or toxic effects) u Understand complex relationships – tolerance, sensitization, mechanistic delay u Explain variability in response u Identify biomarkers and validate surrogate endpoints u Aid dose/dose regimen selection through simulation u Bridge clinical efficacy and safety results across ethnic populations u Bridge clinical results between adult and pediatric patients

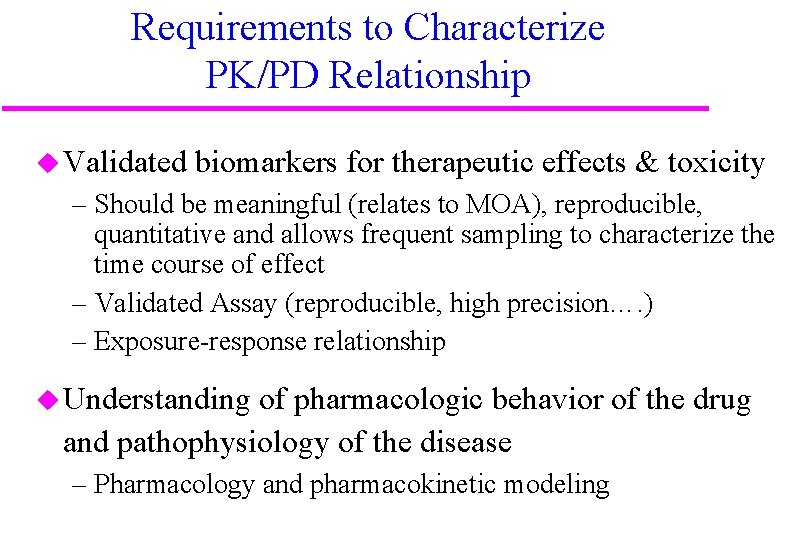
Requirements to Characterize PK/PD Relationship u Validated biomarkers for therapeutic effects & toxicity – Should be meaningful (relates to MOA), reproducible, quantitative and allows frequent sampling to characterize the time course of effect – Validated Assay (reproducible, high precision…. ) – Exposure-response relationship u Understanding of pharmacologic behavior of the drug and pathophysiology of the disease – Pharmacology and pharmacokinetic modeling

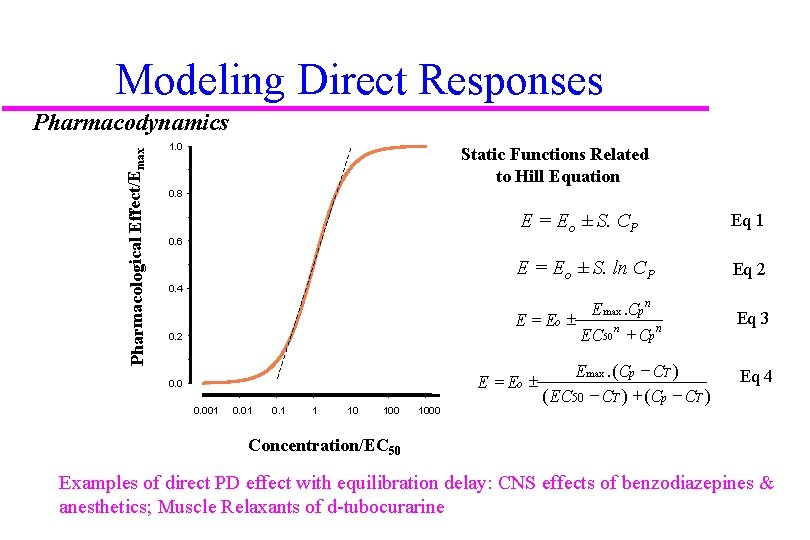
Modeling Direct Responses Pharmacological Effect/Emax Pharmacodynamics 1. 0 Static Functions Related to Hill Equation 0. 8 E = Eo ± S. CP Eq 1 E = Eo ± S. ln CP Eq 2 E max. Cp n E = Eo ± EC 50 n + Cp n Eq 3 0. 6 0. 4 0. 2 E = Eo ± 0. 001 0. 1 1 10 1000 E max. (Cp - CT ) ( EC 50 - CT ) + (Cp - CT ) Eq 4 Concentration/EC 50 Examples of direct PD effect with equilibration delay: CNS effects of benzodiazepines & anesthetics; Muscle Relaxants of d-tubocurarine

Complexities in PK/PD Modeling u Equilibration u Mechanistic delay u Tolerance u Sensitization u Active u Drug metabolites interaction

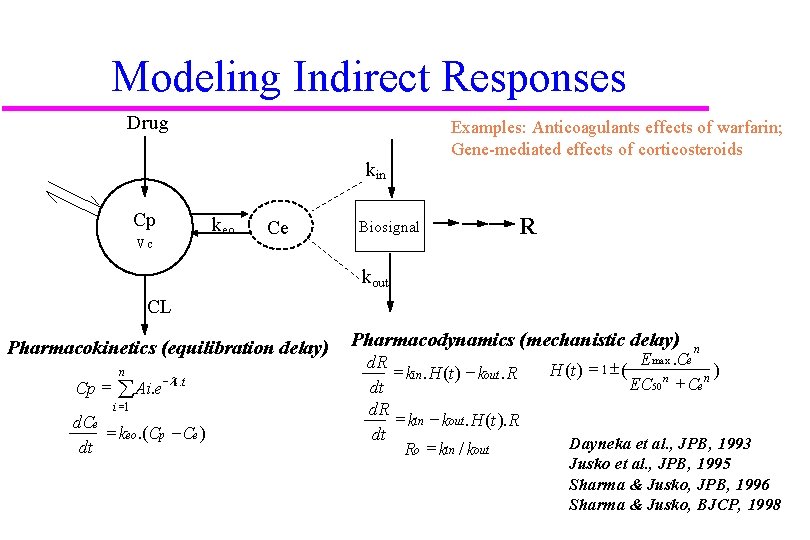
Modeling Indirect Responses Drug kin Cp Vc keo Ce Examples: Anticoagulants effects of warfarin; Gene-mediated effects of corticosteroids Biosignal R kout CL Pharmacokinetics (equilibration delay) Cp = n åAi. e-li. t i =1 d. Ce = keo. (Cp - Ce ) dt Pharmacodynamics (mechanistic delay) d. R = kin. H (t ) - kout. R dt d. R = kin - kout. H (t ). R dt Ro = kin / kout n E max. Ce H (t ) = 1 ± ( ) n n EC 50 + Ce Dayneka et al. , JPB, 1993 Jusko et al. , JPB, 1995 Sharma & Jusko, JPB, 1996 Sharma & Jusko, BJCP, 1998

Examples u IL 12: Tolerance in efficacy & safety biomarker response (IFNg). u CD 4 m. Abs: Validate a safety biomarker in the preclinical transgenic mice model. u IL 5 m. Ab: Biomarker (eosinophil) is not a validated surrogate endpoint. u P 38 MAPK: Characterize an experimental model of acute inflammation for anti-TNF response. u Avitriptan: u Pop Characterize safety profile (BP and heart rate). PK/PD approach in Linezolid bridging program.

IL 12: An example of complex PK/PD relationship

IL 12 u. A 70 k. Da heterodimer cytokine (35+40 k. Da subunits). u Enhances T helper 1 -type immunity. u Potentiates secretion of IFNg by, and the cytolytic activity of, NK cells and CTLs. u IL 12 -induced secretion of IFNg is required for activity. u m. IL 12 has potent antitumor& antimetastatic activity in murine tumor models. u Under development for cancer and infectious diseases.

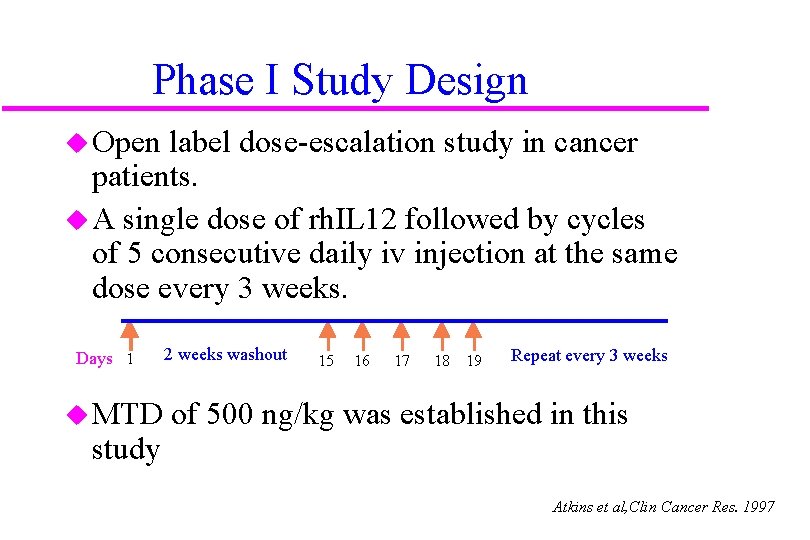
Phase I Study Design u Open label dose-escalation study in cancer patients. u A single dose of rh. IL 12 followed by cycles of 5 consecutive daily iv injection at the same dose every 3 weeks. Days 1 u MTD study 2 weeks washout 15 16 17 18 19 Repeat every 3 weeks of 500 ng/kg was established in this Atkins et al, Clin Cancer Res. 1997

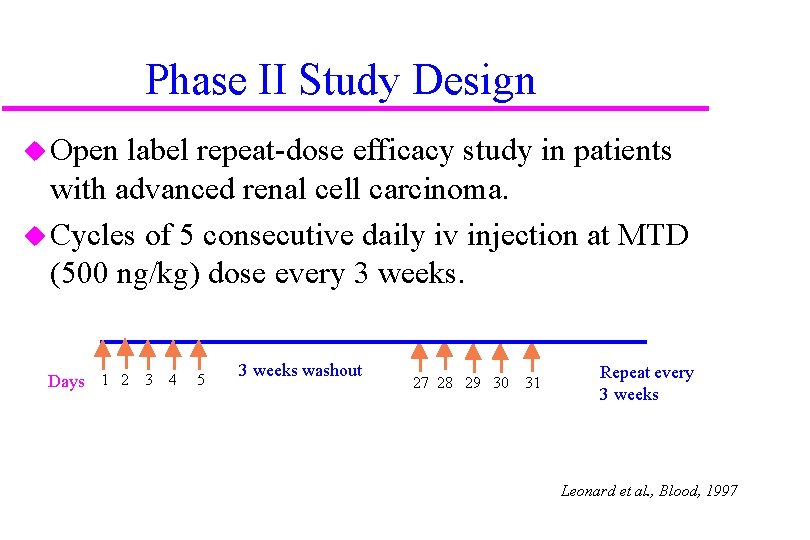
Phase II Study Design u Open label repeat-dose efficacy study in patients with advanced renal cell carcinoma. u Cycles of 5 consecutive daily iv injection at MTD (500 ng/kg) dose every 3 weeks. Days 1 2 3 4 5 3 weeks washout 27 28 29 30 31 Repeat every 3 weeks Leonard et al. , Blood, 1997

Phase II Study Results u Treatment was associated with unexpected serious adverse events. u Most of the patients experienced serious AEs after 2 nd and 3 rd doses. u Two patients died and no one entered the 2 nd cycle due to drug related toxicity such as GI bleeding. u PK profiles for IL 12 were comparable to those observed in Phase I study. Leonard et al. , Blood, 1997

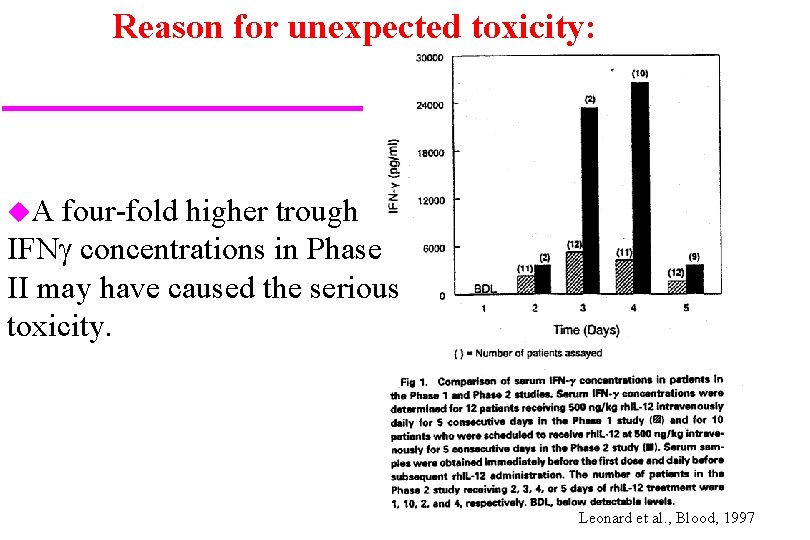
Reason for unexpected toxicity: u. A four-fold higher trough IFNg concentrations in Phase II may have caused the serious toxicity. Leonard et al. , Blood, 1997

Summary u If IFNg concentrations were used as a safety biomarker, it would have been possible to avoid serious AEs by stopping after 2 nd dose in Phase II study. u A single dose of IL 12 causes tolerance in its ability to induce IFNg production upon further dosing. u IL 12 produces tolerance rapidly (3 -4 days) during multiple dosing which lasts for a relatively long time period ( 14 days) in humans. u PK/PD modeling to characterize schedule-dependent IL 12 -induced IFNg production is crucial for designing safe and effective dosing regimens.

Comparative PD of Anti-CD 4 m. Abs in Transgenic Mice Sharma et al. , JPET, 2000

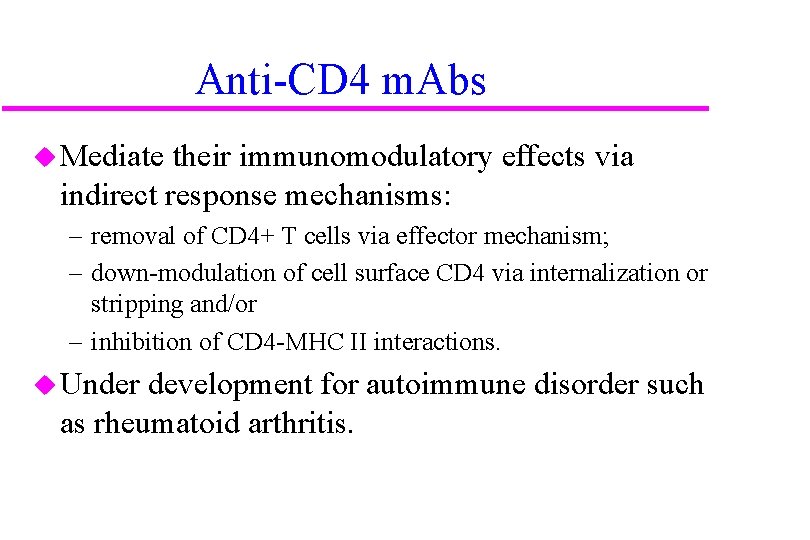
Anti-CD 4 m. Abs u Mediate their immunomodulatory effects via indirect response mechanisms: – removal of CD 4+ T cells via effector mechanism; – down-modulation of cell surface CD 4 via internalization or stripping and/or – inhibition of CD 4 -MHC II interactions. u Under development for autoimmune disorder such as rheumatoid arthritis.

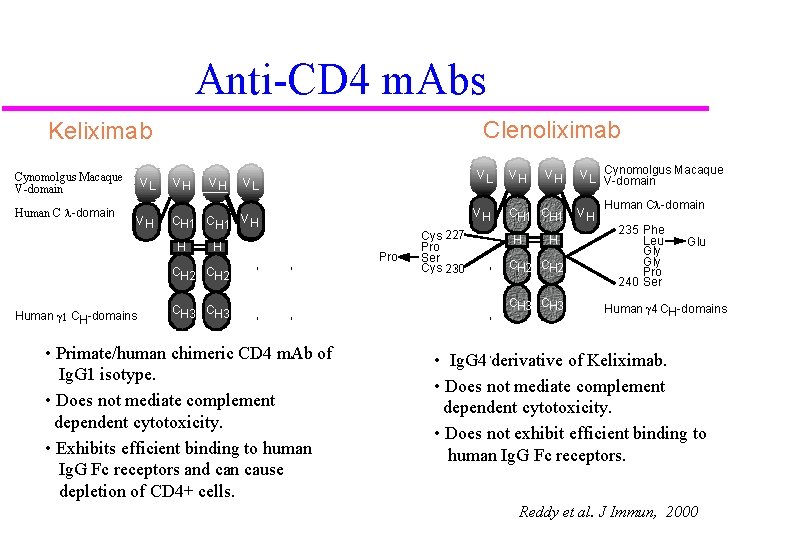
Anti-CD 4 m. Abs Clenoliximab Keliximab Cynomolgus Macaque V-domain Human C l-domain VL VH VH CH 1 C H 1 V H H H CH 2 Human g 1 CH-domains VL VL CH 3 • Primate/human chimeric CD 4 m. Ab of Ig. G 1 isotype. • Does not mediate complement dependent cytotoxicity. • Exhibits efficient binding to human Ig. G Fc receptors and can cause depletion of CD 4+ cells. Pro Cys 227 Pro Ser Cys 230 VH VH CH 1 H H CH 2 CH 3 V L Cynomolgus Macaque V-domain VH Human Cl-domain 235 Phe Leu Gly Pro 240 Ser Glu Human g 4 CH-domains • Ig. G 4 derivative of Keliximab. • Does not mediate complement dependent cytotoxicity. • Does not exhibit efficient binding to human Ig. G Fc receptors. Reddy et al. J Immun, 2000

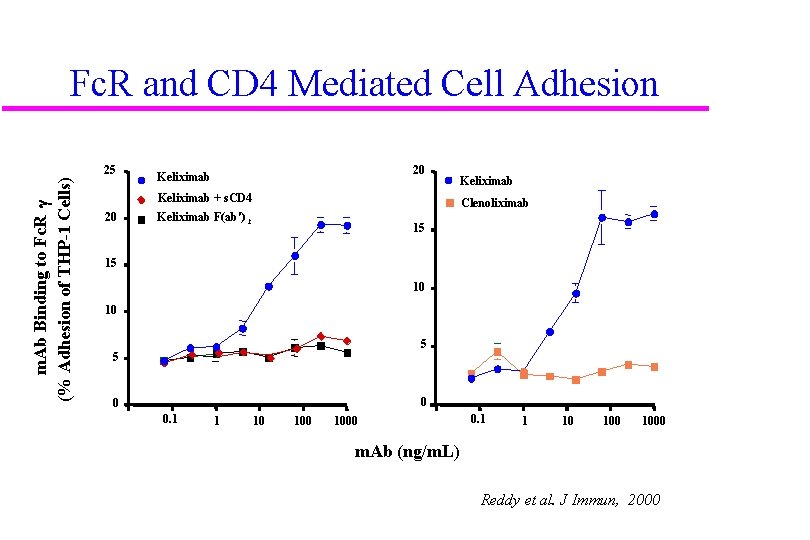
Fc. R and CD 4 Mediated Cell Adhesion m. Ab Binding to Fc. R g (% Adhesion of THP-1 Cells) 25 20 Keliximab + s. CD 4 20 Clenoliximab Keliximab F(ab') 2 15 15 10 10 5 5 0 0 0. 1 1 10 100 1000 m. Ab (ng/m. L) Reddy et al. J Immun, 2000

Study Design u Male transgenic mice (n=10 -13 per group) bearing human CD 4 in place of the mouse CD 4. u Three dose levels (5, 25 & 125 mg/kg). u PK: unbound plasma m. Ab concentrations. u PD: CD 4+ T cells; number of CD 4 epitopes on the surface of T cells and CD 8+ T cells.

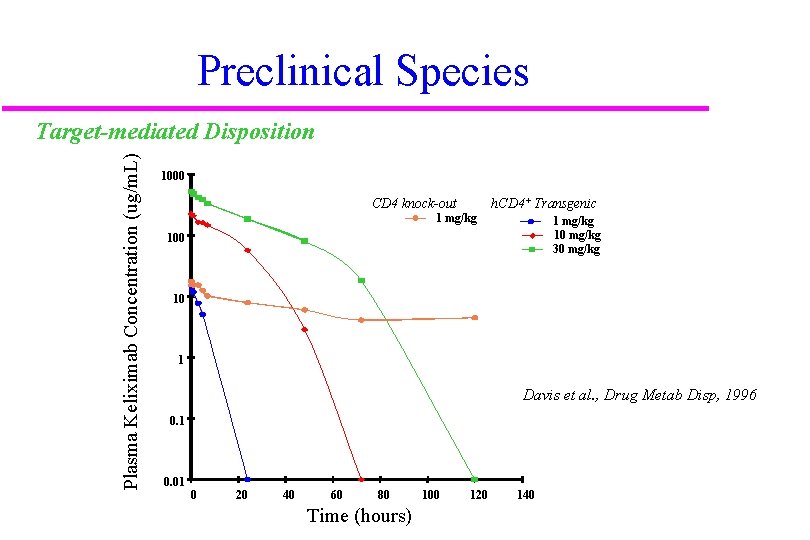
Preclinical Species Plasma Keliximab Concentration (ug/m. L) Target-mediated Disposition 1000 CD 4 knock-out 1 mg/kg h. CD 4+ Transgenic 1 mg/kg 10 mg/kg 30 mg/kg 100 10 1 Davis et al. , Drug Metab Disp, 1996 0. 1 0. 01 0 20 40 60 80 Time (hours) 100 120 140

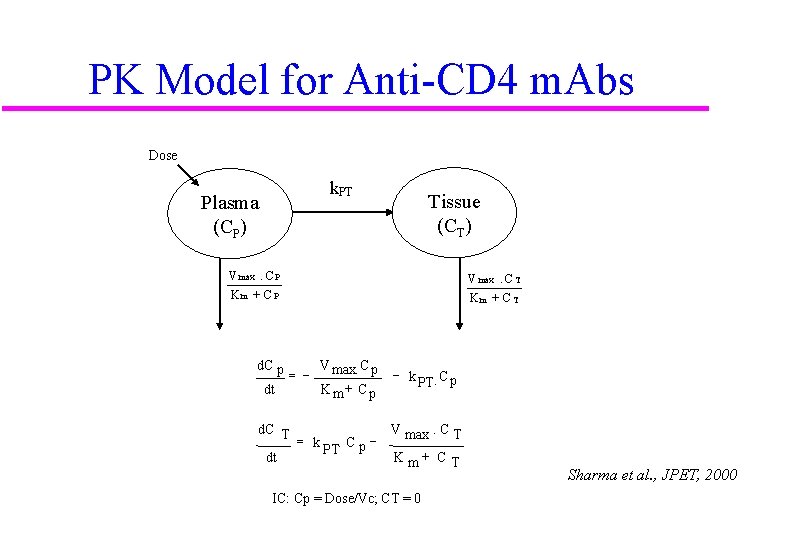
PK Model for Anti-CD 4 m. Abs Dose k. PT Plasma (CP) Tissue (CT) V max. C P V max. C T Km + CP Km + CT d. C p dt = - V max. C p Km+ Cp - k. C p PT d. C T V max. C T = k. Cp. PT dt Km+ CT IC: Cp = Dose/Vc; CT = 0 Sharma et al. , JPET, 2000

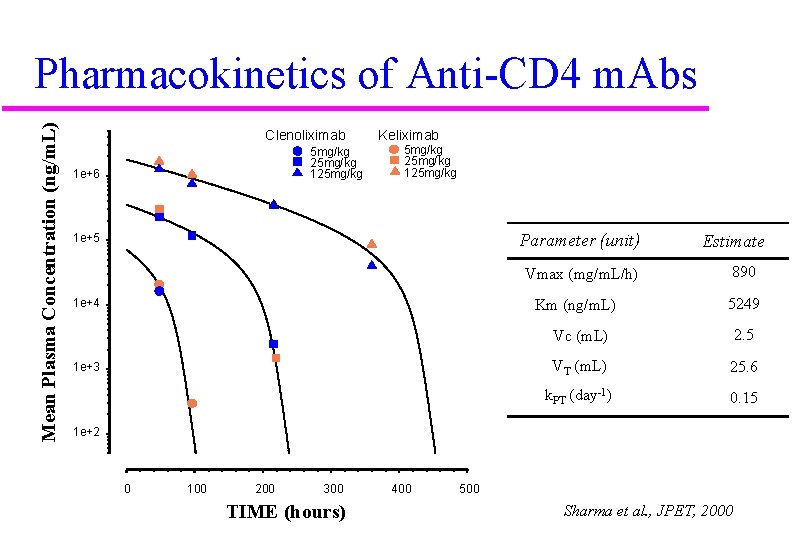
Mean Plasma Concentration (ng/m. L) Pharmacokinetics of Anti-CD 4 m. Abs Clenoliximab 5 mg/kg 25 mg/kg 1 e+6 Keliximab 5 mg/kg 25 mg/kg 125 mg/kg Parameter (unit) 1 e+5 Vmax (mg/m. L/h) 1 e+4 Km (ng/m. L) 1 e+3 Estimate 890 5249 Vc (m. L) 2. 5 VT (m. L) 25. 6 k. PT (day-1) 0. 15 1 e+2 0 100 200 300 TIME (hours) 400 500 Sharma et al. , JPET, 2000

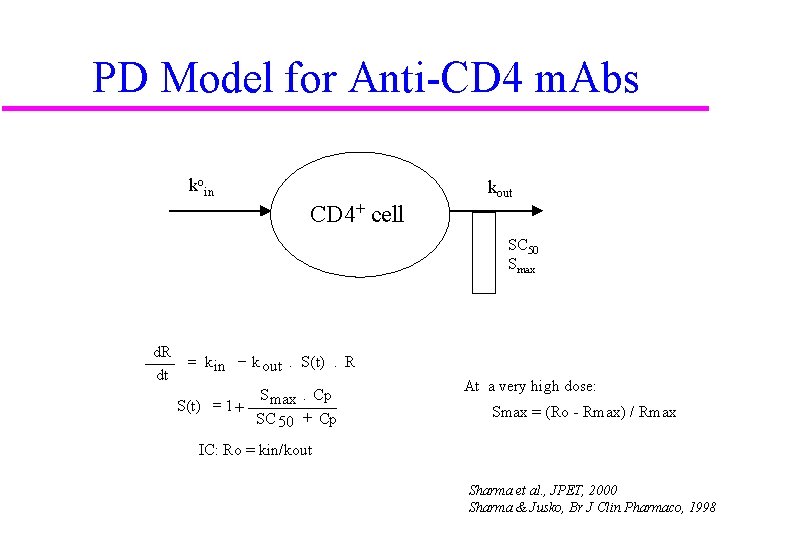
PD Model for Anti-CD 4 m. Abs koin kout CD 4+ cell SC 50 Smax d. R dt = k in - k out. S(t). R S max. Cp S(t) = 1 + SC 50 + Cp At a very high dose: Smax = (Ro - Rmax) / Rmax IC: Ro = kin/kout Sharma et al. , JPET, 2000 Sharma & Jusko, Br J Clin Pharmaco, 1998

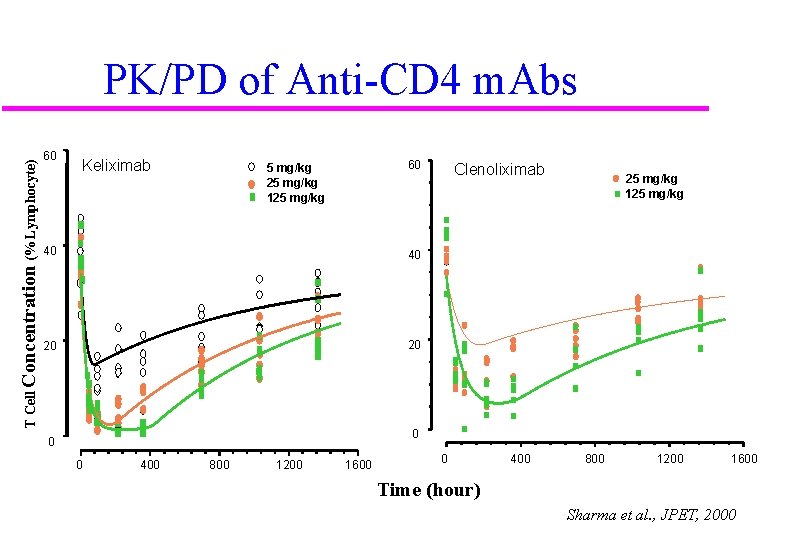
T Cell Concentration (% Lymphocyte) PK/PD of Anti-CD 4 m. Abs 60 Keliximab 60 5 mg/kg 25 mg/kg 125 mg/kg 40 40 20 20 Clenoliximab 25 mg/kg 125 mg/kg 0 0 0 400 800 1200 1600 Time (hour) Sharma et al. , JPET, 2000

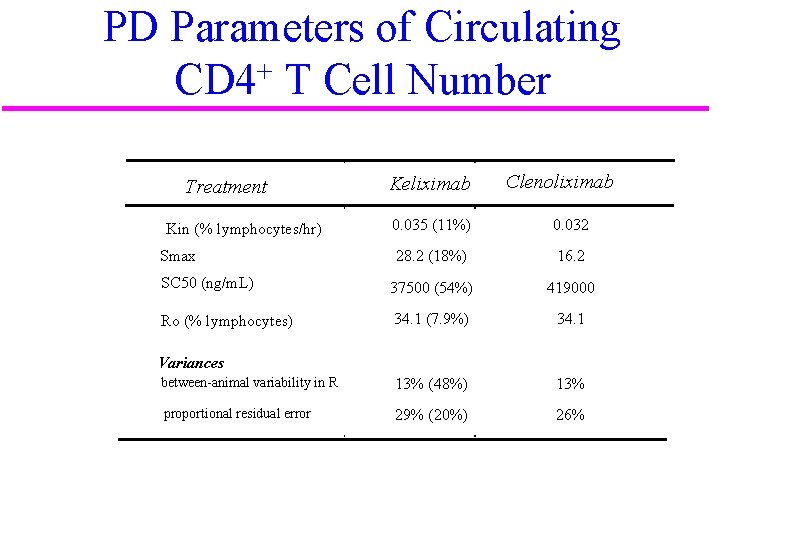
PD Parameters of Circulating CD 4+ T Cell Number Treatment Keliximab Clenoliximab 0. 035 (11%) 0. 032 28. 2 (18%) 16. 2 SC 50 (ng/m. L) 37500 (54%) 419000 Ro (% lymphocytes) 34. 1 (7. 9%) 34. 1 between-animal variability in R 13% (48%) 13% proportional residual error 29% (20%) 26% Kin (% lymphocytes/hr) Smax Variances

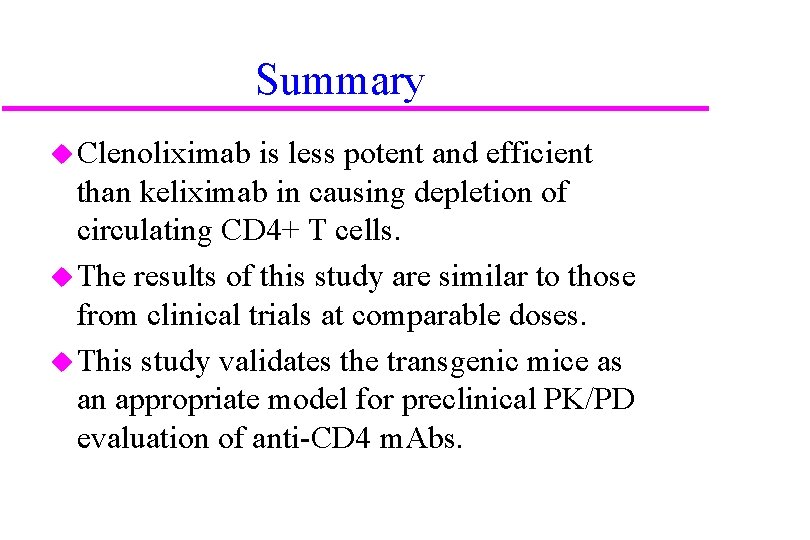
Summary u Clenoliximab is less potent and efficient than keliximab in causing depletion of circulating CD 4+ T cells. u The results of this study are similar to those from clinical trials at comparable doses. u This study validates the transgenic mice as an appropriate model for preclinical PK/PD evaluation of anti-CD 4 m. Abs.