Application of phase diagrams in development of transparent

- Slides: 18

Application of phase diagrams in development of transparent polycrystalline oxides and thermal barrier coating Phase diagrams for production of transparent polycrystalline materials Phase diagrams for thermal barrier coating

Phase chemistry in the development of transparent polycrystalline oxides Polycrystalline oxides are used for optical applications. The Al 2 O 3 is a key element in high pressure sodium lamp. Number of oxides have been developed for optical applications. They are Al 2 O 3, Mg. Al 2 O 4, Al. ON, Y 2 O 3. The aim is to obtain transparent polycrystalline single phase without porosity. 1. Y 2 O 3 High melting point and capacity for wideband transmittance of Y 2 O 3 presents interest for many infrared applications. Transparent Y 2 O 3 -Gd 2 O 3 are unique X-ray scintillators. Pure un-doped Y 2 O 3 can be fabricated to transparency by employing combination of fine active powders and high – temperature press forging and hot isostatic pressing. Success of these approaches relies not only on powder properties and application of pressure to enhance densification kinetics, but also on high purity to prevent precipitation of second phases or coloration from transitions or rare earth ions. Phase relations become critical when pressure-less method of sintering is selected to attain transparency. The options are liquid phase sintering, transient second phase sintering and doped solid state sintering. Non coloring aliovalent sintering aids for Y 2 O 3 among rare earths is restricted to La and Gd, because these ions have no electronic transitions in the visible or infrared frequencies.

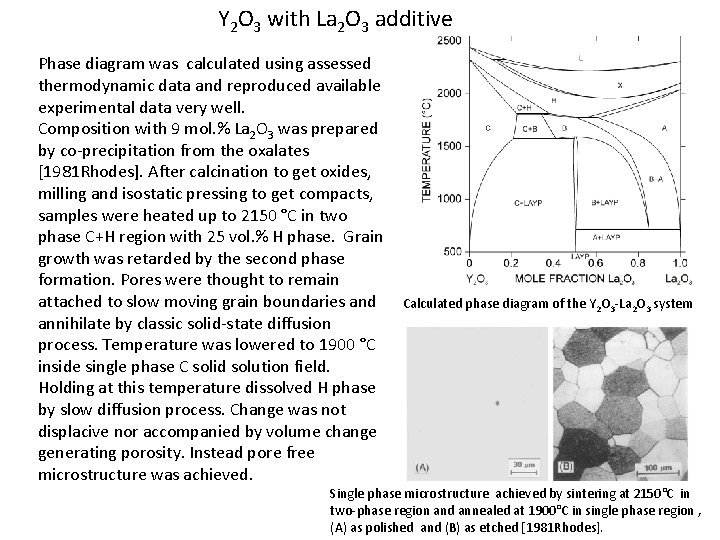
Y 2 O 3 with La 2 O 3 additive Phase diagram was calculated using assessed thermodynamic data and reproduced available experimental data very well. Composition with 9 mol. % La 2 O 3 was prepared by co-precipitation from the oxalates [1981 Rhodes]. After calcination to get oxides, milling and isostatic pressing to get compacts, samples were heated up to 2150 °C in two phase C+H region with 25 vol. % H phase. Grain growth was retarded by the second phase formation. Pores were thought to remain attached to slow moving grain boundaries and annihilate by classic solid-state diffusion process. Temperature was lowered to 1900 °C inside single phase C solid solution field. Holding at this temperature dissolved H phase by slow diffusion process. Change was not displacive nor accompanied by volume change generating porosity. Instead pore free microstructure was achieved. Calculated phase diagram of the Y 2 O 3 -La 2 O 3 system Single phase microstructure achieved by sintering at 2150°C in two-phase region and annealed at 1900°C in single phase region , (A) as polished and (B) as etched [1981 Rhodes].

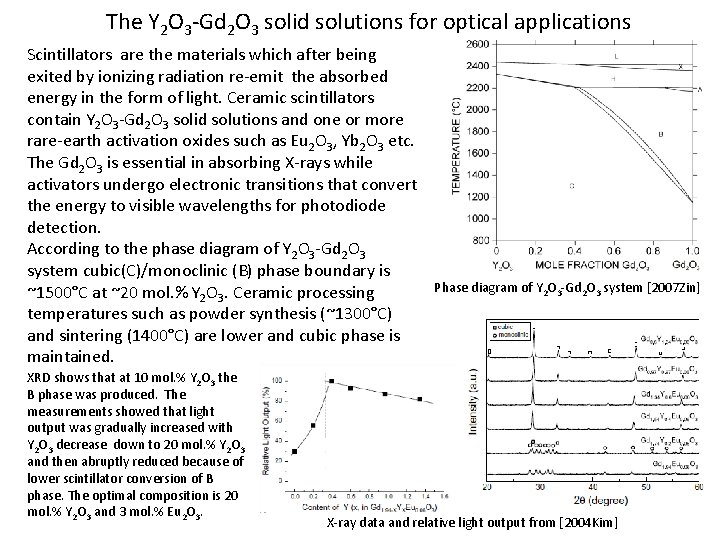
The Y 2 O 3 -Gd 2 O 3 solid solutions for optical applications Scintillators are the materials which after being exited by ionizing radiation re-emit the absorbed energy in the form of light. Ceramic scintillators contain Y 2 O 3 -Gd 2 O 3 solid solutions and one or more rare-earth activation oxides such as Eu 2 O 3, Yb 2 O 3 etc. The Gd 2 O 3 is essential in absorbing X-rays while activators undergo electronic transitions that convert the energy to visible wavelengths for photodiode detection. According to the phase diagram of Y 2 O 3 -Gd 2 O 3 system cubic(C)/monoclinic (B) phase boundary is ~1500°C at ~20 mol. % Y 2 O 3. Ceramic processing temperatures such as powder synthesis (~1300°C) and sintering (1400°C) are lower and cubic phase is maintained. XRD shows that at 10 mol. % Y 2 O 3 the B phase was produced. The measurements showed that light output was gradually increased with Y 2 O 3 decrease down to 20 mol. % Y 2 O 3 and then abruptly reduced because of lower scintillator conversion of B phase. The optimal composition is 20 mol. % Y 2 O 3 and 3 mol. % Eu 2 O 3. Phase diagram of Y 2 O 3 -Gd 2 O 3 system [2007 Zin] X-ray data and relative light output from [2004 Kim]

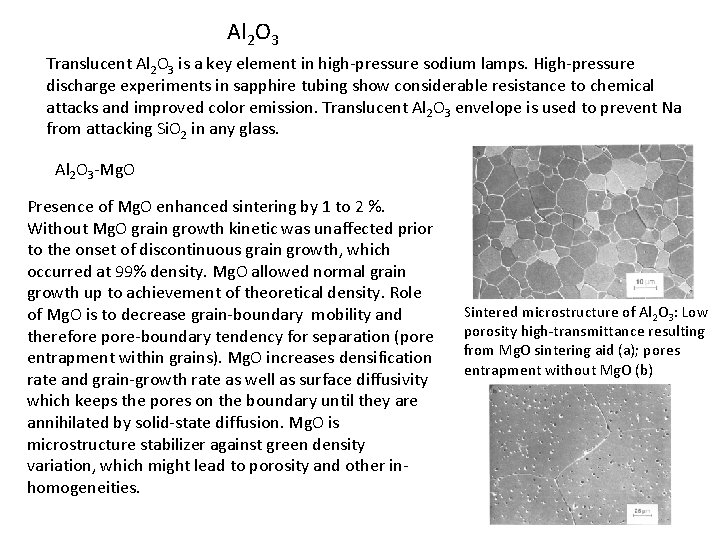
Al 2 O 3 Translucent Al 2 O 3 is a key element in high-pressure sodium lamps. High-pressure discharge experiments in sapphire tubing show considerable resistance to chemical attacks and improved color emission. Translucent Al 2 O 3 envelope is used to prevent Na from attacking Si. O 2 in any glass. Al 2 O 3 -Mg. O Presence of Mg. O enhanced sintering by 1 to 2 %. Without Mg. O grain growth kinetic was unaffected prior to the onset of discontinuous grain growth, which occurred at 99% density. Mg. O allowed normal grain growth up to achievement of theoretical density. Role of Mg. O is to decrease grain-boundary mobility and therefore pore-boundary tendency for separation (pore entrapment within grains). Mg. O increases densification rate and grain-growth rate as well as surface diffusivity which keeps the pores on the boundary until they are annihilated by solid-state diffusion. Mg. O is microstructure stabilizer against green density variation, which might lead to porosity and other inhomogeneities. Sintered microstructure of Al 2 O 3: Low porosity high-transmittance resulting from Mg. O sintering aid (a); pores entrapment without Mg. O (b)

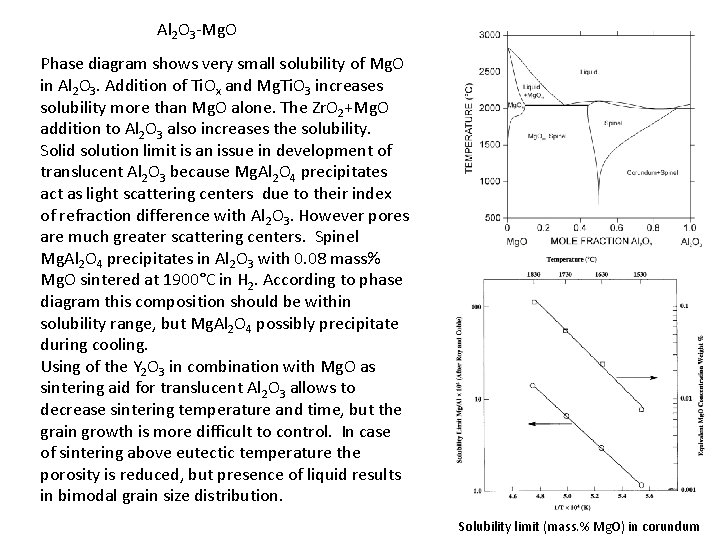
Al 2 O 3 -Mg. O Phase diagram shows very small solubility of Mg. O in Al 2 O 3. Addition of Ti. Ox and Mg. Ti. O 3 increases solubility more than Mg. O alone. The Zr. O 2+Mg. O addition to Al 2 O 3 also increases the solubility. Solid solution limit is an issue in development of translucent Al 2 O 3 because Mg. Al 2 O 4 precipitates act as light scattering centers due to their index of refraction difference with Al 2 O 3. However pores are much greater scattering centers. Spinel Mg. Al 2 O 4 precipitates in Al 2 O 3 with 0. 08 mass% Mg. O sintered at 1900°C in H 2. According to phase diagram this composition should be within solubility range, but Mg. Al 2 O 4 possibly precipitate during cooling. Using of the Y 2 O 3 in combination with Mg. O as sintering aid for translucent Al 2 O 3 allows to decrease sintering temperature and time, but the grain growth is more difficult to control. In case of sintering above eutectic temperature the porosity is reduced, but presence of liquid results in bimodal grain size distribution. Solubility limit (mass. % Mg. O) in corundum

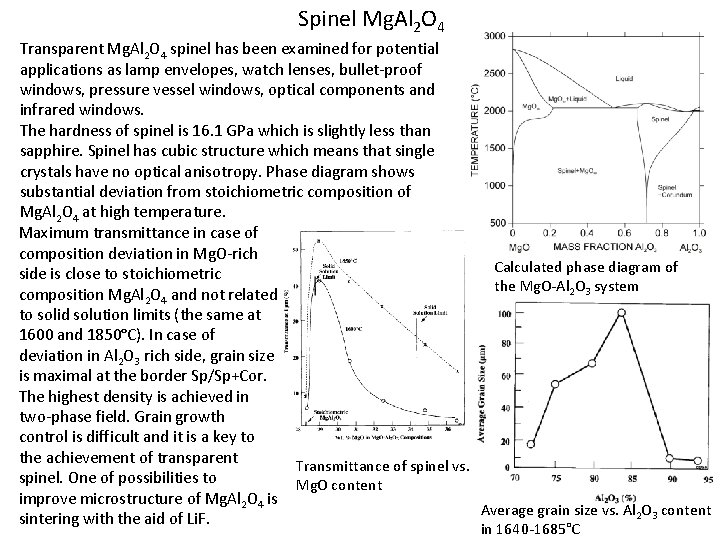
Spinel Mg. Al 2 O 4 Transparent Mg. Al 2 O 4 spinel has been examined for potential applications as lamp envelopes, watch lenses, bullet-proof windows, pressure vessel windows, optical components and infrared windows. The hardness of spinel is 16. 1 GPa which is slightly less than sapphire. Spinel has cubic structure which means that single crystals have no optical anisotropy. Phase diagram shows substantial deviation from stoichiometric composition of Mg. Al 2 O 4 at high temperature. Maximum transmittance in case of composition deviation in Mg. O-rich Calculated phase diagram of side is close to stoichiometric the Mg. O-Al 2 O 3 system composition Mg. Al 2 O 4 and not related to solid solution limits (the same at 1600 and 1850°C). In case of deviation in Al 2 O 3 rich side, grain size is maximal at the border Sp/Sp+Cor. The highest density is achieved in two-phase field. Grain growth control is difficult and it is a key to the achievement of transparent Transmittance of spinel vs. spinel. One of possibilities to Mg. O content improve microstructure of Mg. Al 2 O 4 is Average grain size vs. Al 2 O 3 content sintering with the aid of Li. F. in 1640 -1685°C

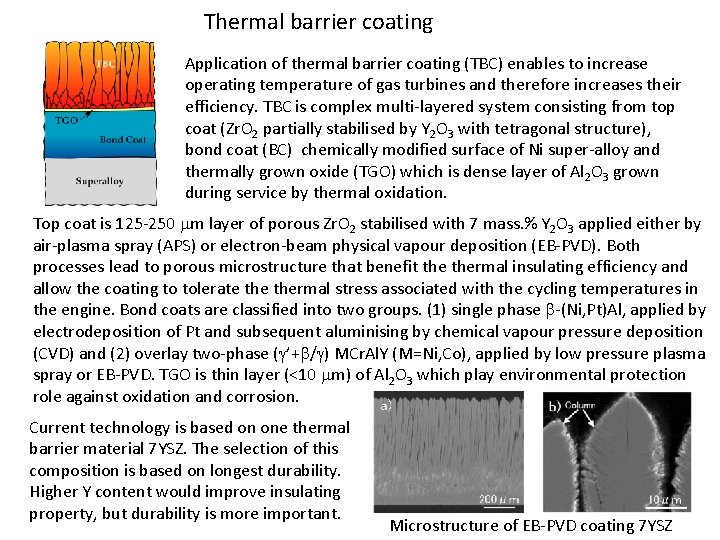
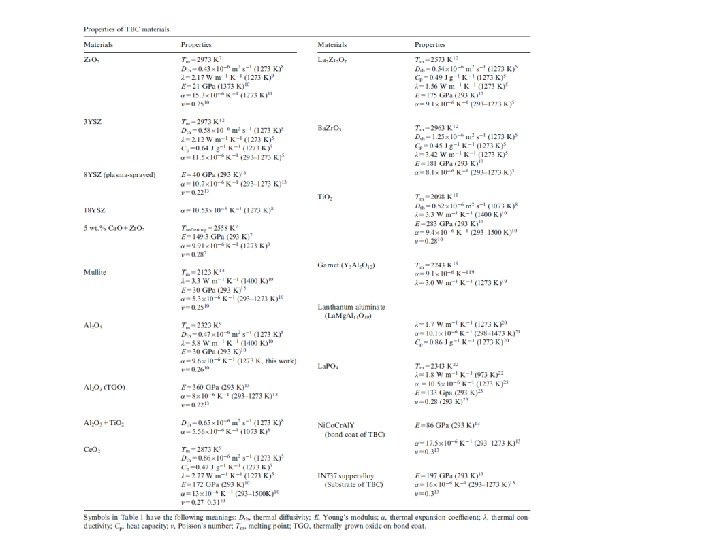
Thermal barrier coating Application of thermal barrier coating (TBC) enables to increase operating temperature of gas turbines and therefore increases their efficiency. TBC is complex multi-layered system consisting from top coat (Zr. O 2 partially stabilised by Y 2 O 3 with tetragonal structure), bond coat (BC) chemically modified surface of Ni super-alloy and thermally grown oxide (TGO) which is dense layer of Al 2 O 3 grown during service by thermal oxidation. Top coat is 125 -250 mm layer of porous Zr. O 2 stabilised with 7 mass. % Y 2 O 3 applied either by air-plasma spray (APS) or electron-beam physical vapour deposition (EB-PVD). Both processes lead to porous microstructure that benefit thermal insulating efficiency and allow the coating to tolerate thermal stress associated with the cycling temperatures in the engine. Bond coats are classified into two groups. (1) single phase b-(Ni, Pt)Al, applied by electrodeposition of Pt and subsequent aluminising by chemical vapour pressure deposition (CVD) and (2) overlay two-phase (g’+b/g) MCr. Al. Y (M=Ni, Co), applied by low pressure plasma spray or EB-PVD. TGO is thin layer (<10 mm) of Al 2 O 3 which play environmental protection role against oxidation and corrosion. Current technology is based on one thermal barrier material 7 YSZ. The selection of this composition is based on longest durability. Higher Y content would improve insulating property, but durability is more important. Microstructure of EB-PVD coating 7 YSZ

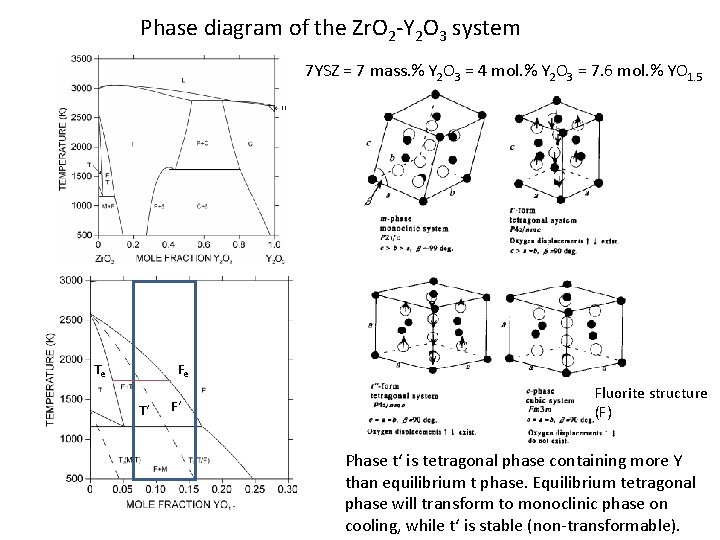
Phase diagram of the Zr. O 2 -Y 2 O 3 system 7 YSZ = 7 mass. % Y 2 O 3 = 4 mol. % Y 2 O 3 = 7. 6 mol. % YO 1. 5 Te Fe T‘ F‘ Fluorite structure (F) Phase t‘ is tetragonal phase containing more Y than equilibrium t phase. Equilibrium tetragonal phase will transform to monoclinic phase on cooling, while t‘ is stable (non-transformable).

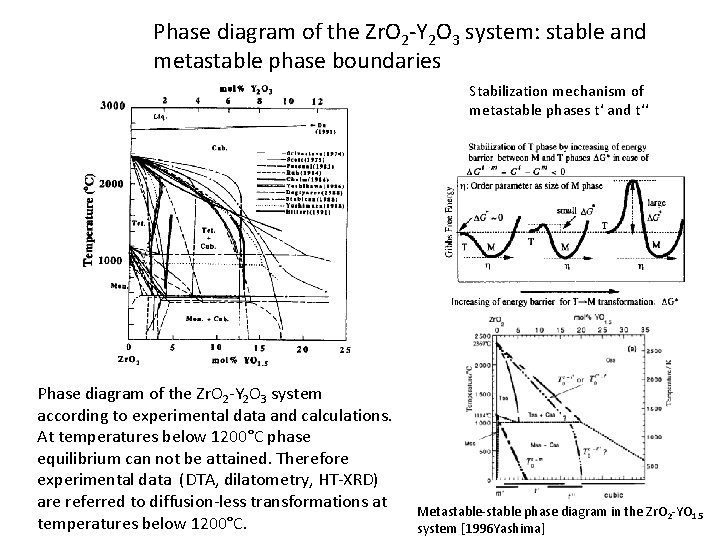
Phase diagram of the Zr. O 2 -Y 2 O 3 system: stable and metastable phase boundaries Stabilization mechanism of metastable phases t‘ and t‘‘ Phase diagram of the Zr. O 2 -Y 2 O 3 system according to experimental data and calculations. At temperatures below 1200°C phase equilibrium can not be attained. Therefore experimental data (DTA, dilatometry, HT-XRD) are referred to diffusion-less transformations at temperatures below 1200°C. Metastable-stable phase diagram in the Zr. O 2 -YO 1. 5 system [1996 Yashima]

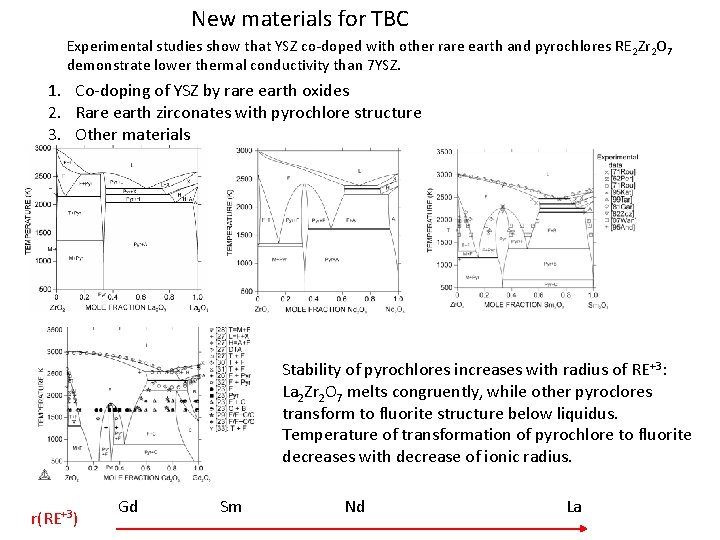
New materials for TBC Experimental studies show that YSZ co-doped with other rare earth and pyrochlores RE 2 Zr 2 O 7 demonstrate lower thermal conductivity than 7 YSZ. 1. Co-doping of YSZ by rare earth oxides 2. Rare earth zirconates with pyrochlore structure 3. Other materials Stability of pyrochlores increases with radius of RE+3: La 2 Zr 2 O 7 melts congruently, while other pyroclores transform to fluorite structure below liquidus. Temperature of transformation of pyrochlore to fluorite decreases with decrease of ionic radius. r(RE+3) Gd Sm Nd La

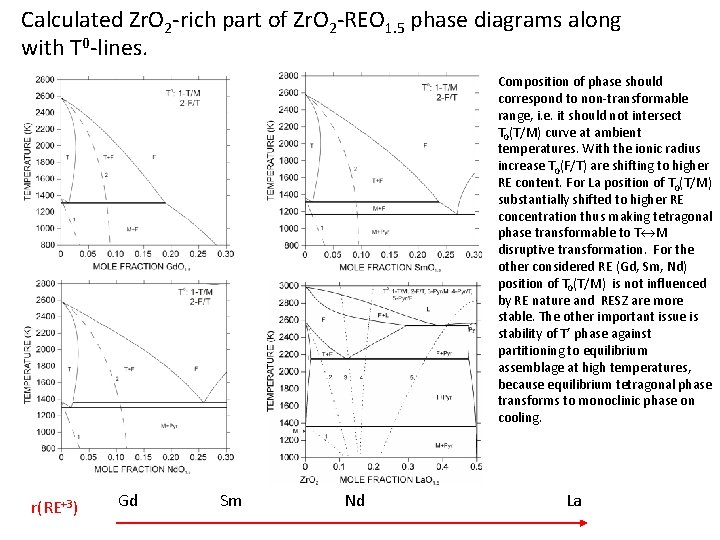
Calculated Zr. O 2 -rich part of Zr. O 2 -REO 1. 5 phase diagrams along with T 0 -lines. Composition of phase should correspond to non-transformable range, i. e. it should not intersect T 0(T/M) curve at ambient temperatures. With the ionic radius increase T 0(F/T) are shifting to higher RE content. For La position of T 0(T/M) substantially shifted to higher RE concentration thus making tetragonal phase transformable to T M disruptive transformation. For the other considered RE (Gd, Sm, Nd) position of T 0(T/M) is not influenced by RE nature and RESZ are more stable. The other important issue is stability of T’ phase against partitioning to equilibrium assemblage at high temperatures, because equilibrium tetragonal phase transforms to monoclinic phase on cooling. r(RE+3) Gd Sm Nd La

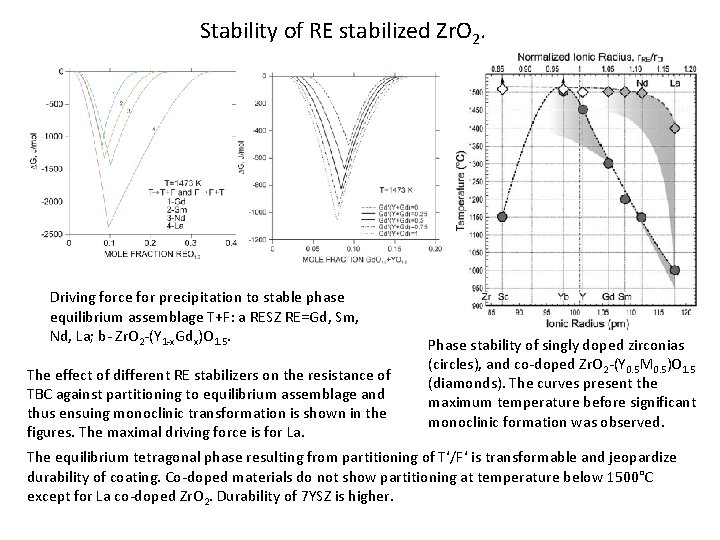
Stability of RE stabilized Zr. O 2. Driving force for precipitation to stable phase equilibrium assemblage T+F: a RESZ RE=Gd, Sm, Nd, La; b- Zr. O 2 -(Y 1 -x. Gdx)O 1. 5. The effect of different RE stabilizers on the resistance of TBC against partitioning to equilibrium assemblage and thus ensuing monoclinic transformation is shown in the figures. The maximal driving force is for La. Phase stability of singly doped zirconias (circles), and co-doped Zr. O 2 -(Y 0. 5 M 0. 5)O 1. 5 (diamonds). The curves present the maximum temperature before significant monoclinic formation was observed. The equilibrium tetragonal phase resulting from partitioning of T‘/F‘ is transformable and jeopardize durability of coating. Co-doped materials do not show partitioning at temperature below 1500°C except for La co-doped Zr. O 2. Durability of 7 YSZ is higher.

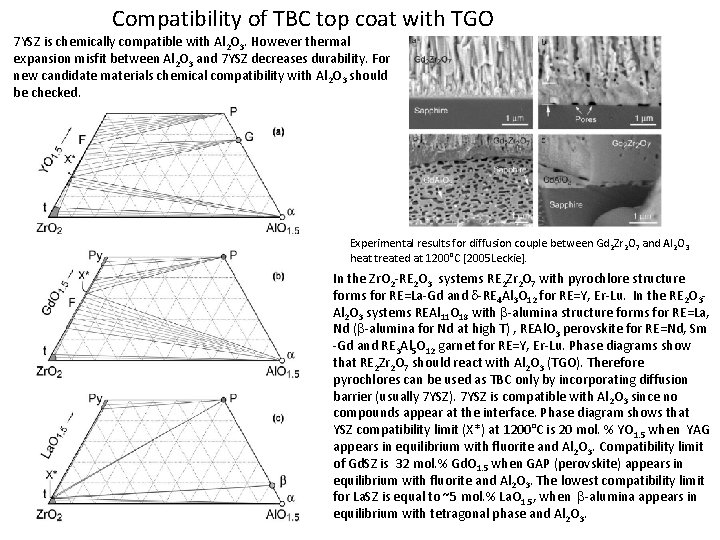
Compatibility of TBC top coat with TGO 7 YSZ is chemically compatible with Al 2 O 3. However thermal expansion misfit between Al 2 O 3 and 7 YSZ decreases durability. For new candidate materials chemical compatibility with Al 2 O 3 should be checked. Experimental results for diffusion couple between Gd 2 Zr 2 O 7 and Al 2 O 3 heat treated at 1200°C [2005 Leckie]. In the Zr. O 2 -RE 2 O 3 systems RE 2 Zr 2 O 7 with pyrochlore structure forms for RE=La-Gd and d-RE 4 Al 3 O 12 for RE=Y, Er-Lu. In the RE 2 O 3 Al 2 O 3 systems REAl 11 O 18 with b-alumina structure forms for RE=La, Nd (b-alumina for Nd at high T) , REAl. O 3 perovskite for RE=Nd, Sm -Gd and RE 3 Al 5 O 12 garnet for RE=Y, Er-Lu. Phase diagrams show that RE 2 Zr 2 O 7 should react with Al 2 O 3 (TGO). Therefore pyrochlores can be used as TBC only by incorporating diffusion barrier (usually 7 YSZ). 7 YSZ is compatible with Al 2 O 3 since no compounds appear at the interface. Phase diagram shows that YSZ compatibility limit (X*) at 1200°C is 20 mol. % YO 1. 5 when YAG appears in equilibrium with fluorite and Al 2 O 3. Compatibility limit of Gd. SZ is 32 mol. % Gd. O 1. 5 when GAP (perovskite) appears in equilibrium with fluorite and Al 2 O 3. The lowest compatibility limit for La. SZ is equal to ~5 mol. % La. O 1. 5, when b-alumina appears in equilibrium with tetragonal phase and Al 2 O 3.

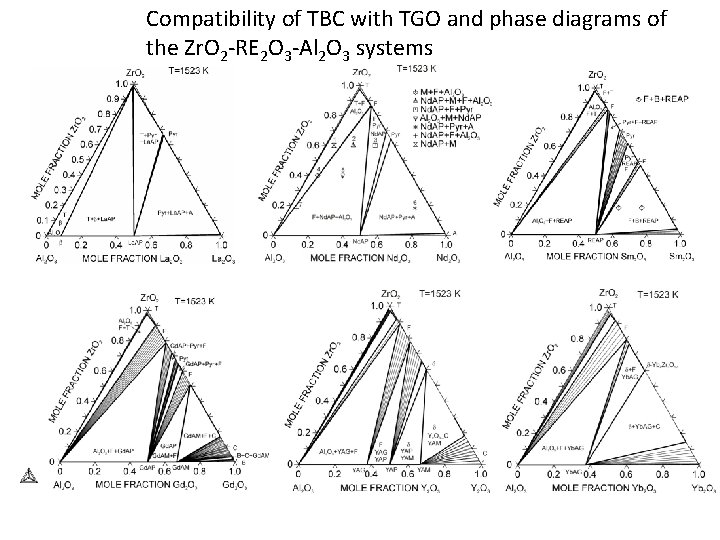
Compatibility of TBC with TGO and phase diagrams of the Zr. O 2 -RE 2 O 3 -Al 2 O 3 systems

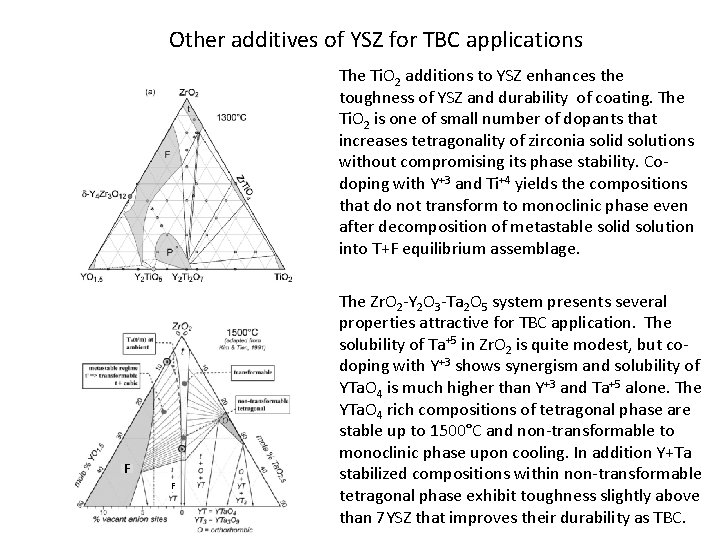
Other additives of YSZ for TBC applications The Ti. O 2 additions to YSZ enhances the toughness of YSZ and durability of coating. The Ti. O 2 is one of small number of dopants that increases tetragonality of zirconia solid solutions without compromising its phase stability. Codoping with Y+3 and Ti+4 yields the compositions that do not transform to monoclinic phase even after decomposition of metastable solid solution into T+F equilibrium assemblage. F F The Zr. O 2 -Y 2 O 3 -Ta 2 O 5 system presents several properties attractive for TBC application. The solubility of Ta+5 in Zr. O 2 is quite modest, but codoping with Y+3 shows synergism and solubility of YTa. O 4 is much higher than Y+3 and Ta+5 alone. The YTa. O 4 rich compositions of tetragonal phase are stable up to 1500°C and non-transformable to monoclinic phase upon cooling. In addition Y+Ta stabilized compositions within non-transformable tetragonal phase exhibit toughness slightly above than 7 YSZ that improves their durability as TBC.


Concluding remarks about novel materials for TBC 7 YSZ is presently used as TBC. The issue is ageing effect on phase stability at temperatures above ~1200°C and necessity to decrease thermal conductivity. The requirements to the new candidate materials are: (1) high melting point; (2) no phase transformations in the range between room temperature and operation temperature; (3) low thermal conductivity; (4) chemical inertness; (5) thermal expansion match with metallic substrate and TGO; (6) good adherence to metallic substrate and TGO; (7) low sintering rate of porous microstructure. To reduce thermal conductivity two groups of candidate materials were considered: RE codoped YSZ and RE zirconates with pyrochlore structure. Multiple co-doping with one smaller cation (Yb, Sc) and one larger (La, Nd, Sm, Gd). The defect nano-cluster system arises which contribute to phonon scattering and reduction of thermal conductivity. Pyrochlores have many advantages such as lower thermal conductivity, high microstructural stability. The disadvantages are low thermal expansion coefficient and therefore larger misfit with TGO and reaction with TGO forming aluminates. The double layer concept can be a solution of these problems: pyrochlore can be used as top layer to reduce thermal conductivity and 7 YSZ as next layer for better compatibility with TGO. Testing of double layer coating indicated better durability than 7 YSZ during cycling at 1300°C [2004 Vassen]. Additional issues arise when considering environmental effect on novel TBC. Many of suggested materials exhibit lower erosion resistance. The other concerns are related to attacks of molten silicates. Molten Ca-Mg-alumo-silicates (CMAS) appear to readily penetrate all Zr. O 2 based compositions. Understanding of reactions between TBC and CMAS is important to develop TBC resistance to CMAS attacks.