Application of Electrochemical Cells Lesson 12 Banana Watch

Application of Electrochemical Cells Lesson 12

Banana Watch
Application of Electrochemical Cells 1. Zn/C or Le. Clanche Cell Anode: Anode Reaction: Zn Zn Cathode: Cathode Reaction: Electrolyte: C Mn 4+ +1 e- → Mn+3 NH 4 Cl and Mn. O 2 Inexpensive → Zn 2+ Not rechargeable + 2 e- Short life
Application of Electrochemical Cell 2. The Alkaline Cell Anode: Anode Reaction: Zn Zn Cathode: Cathode Reaction: Electrolyte: C Mn 4+ +1 e- → Mn 3+ KOH and Mn. O 2 More expensive Not rechargeable → Zn 2+ + 2 e- Longer life
Application of Electrochemical Cells 3. The Lead Acid Battery (Automobile) Anode: Anode Reaction: Cathode Reaction: Electrolyte: Pb Pb → Pb 2+ + 2 e. Pb. O 2 + HSO 4 - + 3 H+ + 2 e- → Pb. SO 4 + 2 H 2 H 2 SO 4 Rechargeable Long life Large current

Application of Electrochemical Cells 4. The Fuel Cell Overall Reaction: Expensive H 2 + ½O 2 → H 2 O + energy Requires fuel Environmentally friendly

Nickel Cadmium Rechargable Cordless Phones

Nickel Metal Hydride Rechargable

Lithium Rechargable Cameras Laptops
Corrosion of Iron Corrosion is oxidation: Fe(s) → Fe 2+ + 2 e- Rust is initially Fe(OH)2 which dries to become Fe 2 O 3. There are three requirements for the corrosion of iron. Iron Water Oxygen Corrosion is spontaneous or an electrochemical cell.
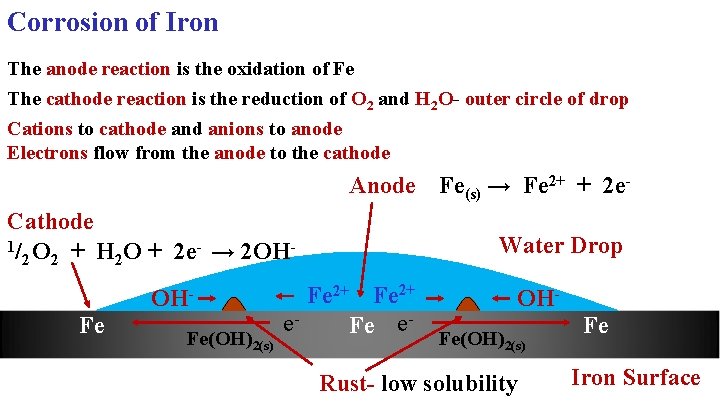
Corrosion of Iron The anode reaction is the oxidation of Fe The cathode reaction is the reduction of O 2 and H 2 O- outer circle of drop Cations to cathode and anions to anode Electrons flow from the anode to the cathode Anode Fe(s) → Fe 2+ + 2 e. Cathode 1/ O + H O + 2 e- → 2 OH 2 2 2 OHFe Fe(OH)2(s) Water Drop Fe 2+ e. Fe e- OHFe(OH)2(s) Rust- low solubility Fe Iron Surface

Methods of Preventing Corrosion Protective Coatings Paint Grease Electroplating Plastic

Cathodic Protection Remember corrosion is oxidation Make iron the cathode of a cell, which is the site of reduction, oxidation cannot occur.
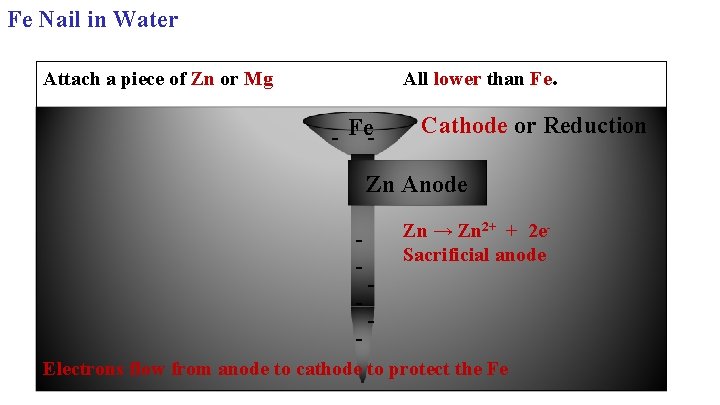
Fe Nail in Water All lower than Fe. Attach a piece of Zn or Mg - Fe- Cathode or Reduction Zn Anode - Zn → Zn 2+ + 2 e. Sacrificial anode Electrons flow from anode to cathode to protect the Fe

Mg bracelets on a ship
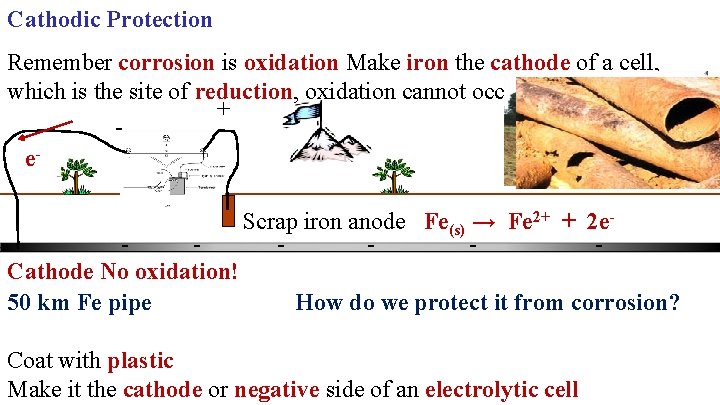
Cathodic Protection Remember corrosion is oxidation Make iron the cathode of a cell, which is the site of reduction, oxidation cannot occur. + e. Cathode No oxidation! 50 km Fe pipe Scrap iron anode Fe(s) → Fe 2+ + 2 e. How do we protect it from corrosion? Coat with plastic Make it the cathode or negative side of an electrolytic cell
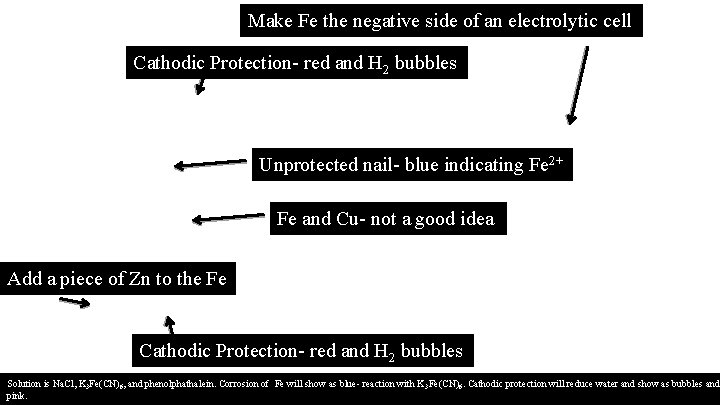
Make Fe the negative side of an electrolytic cell Cathodic Protection- red and H 2 bubbles Unprotected nail- blue indicating Fe 2+ Fe and Cu- not a good idea Add a piece of Zn to the Fe Cathodic Protection- red and H 2 bubbles Solution is Na. Cl, K 3 Fe(CN)6, and phenolphathalein. Corrosion of Fe will show as blue- reaction with K 3 Fe(CN)6. Cathodic protection will reduce water and show as bubbles and pink.

BC Fast Ferry The Aluminum hull is protected by an electrolytic cell When it was first put to sea this system was not in operation. The Paint peeled off requiring a new multimillion dollar paint job.
- Slides: 18