Appendix 1 IMACS Participating Hospitals n 17 Coverage

- Slides: 3

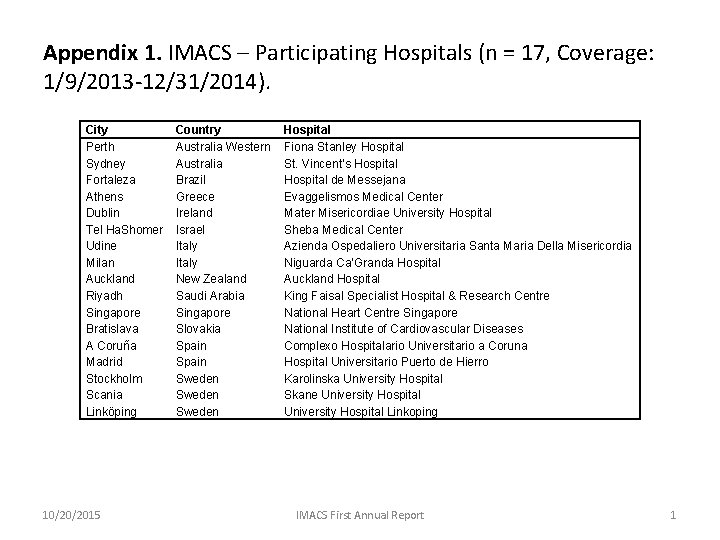
Appendix 1. IMACS – Participating Hospitals (n = 17, Coverage: 1/9/2013 -12/31/2014). City Perth Sydney Fortaleza Athens Dublin Tel Ha. Shomer Udine Milan Auckland Riyadh Singapore Bratislava A Coruña Madrid Stockholm Scania Linköping 10/20/2015 Country Australia Western Australia Brazil Greece Ireland Israel Italy New Zealand Saudi Arabia Singapore Slovakia Spain Sweden Hospital Fiona Stanley Hospital St. Vincent’s Hospital de Messejana Evaggelismos Medical Center Mater Misericordiae University Hospital Sheba Medical Center Azienda Ospedaliero Universitaria Santa Maria Della Misericordia Niguarda Ca’Granda Hospital Auckland Hospital King Faisal Specialist Hospital & Research Centre National Heart Centre Singapore National Institute of Cardiovascular Diseases Complexo Hospitalario Universitario a Coruna Hospital Universitario Puerto de Hierro Karolinska University Hospital Skane University Hospital Linkoping IMACS First Annual Report 1

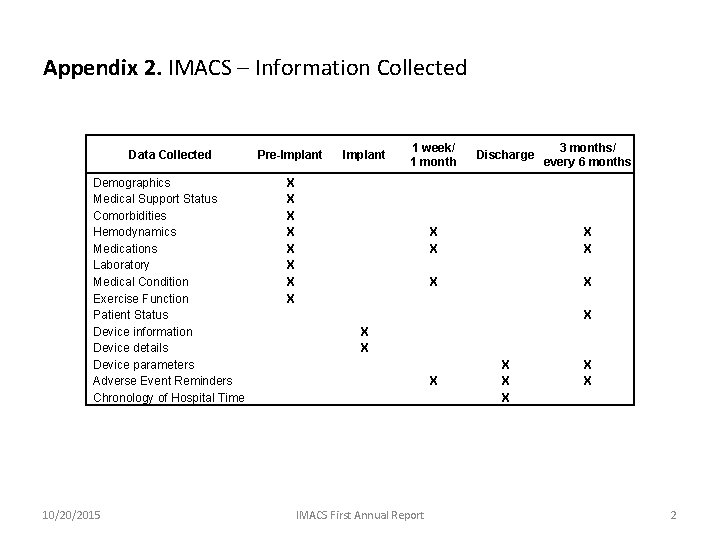
Appendix 2. IMACS – Information Collected Data Collected Pre-Implant 1 week/ 1 month Discharge 3 months/ every 6 months Demographics Medical Support Status Comorbidities Hemodynamics Medications Laboratory Medical Condition Exercise Function Patient Status Device information Device details Device parameters Adverse Event Reminders Chronology of Hospital Time X X X X X X X X X X X X X X X 10/20/2015 IMACS First Annual Report 2

Appendix 3. Potential Covariates used in Multivariable Model Demographics Age, Male, White, College, Married, Body Mass Index , Body Surface Area, Ethnicity Patient Profile Levels NYHA 4 Comorbidities Previous Cancer, Ascites, Diabetes, Smoker, Alcohol Abuse, Cardiovascular accident (CVA), Peripheral vascular disease (PVD), Carotid artery disease, Diagnosis – coronary artery disease, History of CABG, History of Valve surgery, ICD Implant Information Concomitant surgery, Continuous flow device, Bi-ventricular placement, Failure to wean, Post cardiac operation Pre-Implant Laboratory Values Cholesterol, INR, Sodium, Albumin, Total Bilirubi, n SGOT/AST, White blood cell (WBC), Blood urea nitrogen (BUN), Creatinine, Pre-albumin Device Strategy @ Implant Destination Therapy Pre-Implant Interventions Ventilator, IABP, Inotropes, Dialysis Hemodynamics (Pre-Implant) Systolic blood pressure, Diastolic book pressure, Right atrial pressure, Cardiac Output, Cardiac Index, LVEDD%, Pulmonary Wedge pressure, Pulmonary diastolic pressure, Pulmonary systolic pressure, PVR-Cardiac Index, PVR-Cardiac Output, LVEF < 20, Severe RVEF 10/20/2015 IMACS First Annual Report 3