APPEL A PROJET EUROPEEN FP 7 HEALTH2013 innovation















- Slides: 17

APPEL A PROJET EUROPEEN « FP 7 -HEALTH-2013 -innovation 1 FP 7 -HEALTH-2013 -innovation 2» PROJETS COLLABORATIFS

Objective *: “� Improving the health of European citizens and increasing the competitiveness and boosting the innovative capacity of European health-related industries and businesses while addressing global health issues including emerging epidemics. Emphasis will be put on translational research (translation of basic discoveries in clinical applications including scientific validation of experimental results) the development and validation of new therapies, methods for health promotion and prevention including promotion of child health, healthy ageing, diagnostic tools and medical technologies, as well as sustainable and efficient healthcare systems » . do cjoint « FP 7 -health-orientation paper. page 3

The research priorities * for 2013 are: 1 -Brain research. 2 - Antimicrobial drug resistance. 3 -Comparative effectiveness research. 4 Topics from other areas such as: - developing personalised medicines approaches, - cardiovascular research, - safety and efficacy of therapies, - cancer and public health research and -a horizontal activity for translating research results into innovative applications for health. * Doc joint: « FP 7 -health-orientation paper. page 3 à 5 «

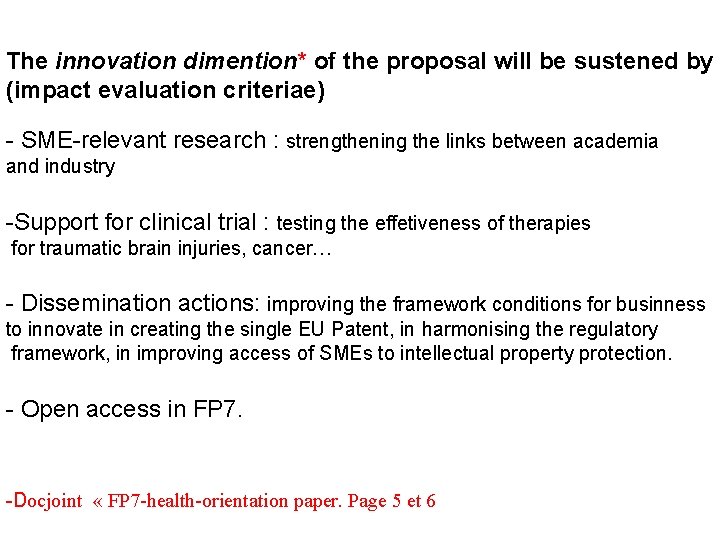
The innovation dimention* of the proposal will be sustened by (impact evaluation criteriae) - SME-relevant research : strengthening the links between academia and industry -Support for clinical trial : testing the effetiveness of therapies for traumatic brain injuries, cancer… - Dissemination actions: improving the framework conditions for businness to innovate in creating the single EU Patent, in harmonising the regulatory framework, in improving access of SMEs to intellectual property protection. - Open access in FP 7. -Docjoint « FP 7 -health-orientation paper. Page 5 et 6

1 Condition générale pour les participation: : Toute entreprise, université, centre de recherche, organisation ou particulier, légalement établi(e) dans un pays quelconque, peut participer à un projet collaboratif (connu sous le nom d’action indirecte) 2 Pays élligibles • Bien que les participants du 7 e PC puissent en principe être basés n’importe où, diverses catégories de pays peuvent disposer d’une éligibilité • ÉTATS MEMBRES – L’UE-27; • PAYS ASSOCIÉS – avec les accords de coopération scientifique et technologique qui ont impliqué la contribution au budget du programme-cadre; • PAYS CANDIDATS – actuellement reconnus en tant que candidats pour une accession future; • PAYS TIERS – la participation d’organisations ou de particuliers établis dans des pays n’étant ni des États membres, ni candidats ni associés, devrait également être justifiée en terme de contribution accrue aux objectifs du 7 e PC. • Visualisez la liste des pays partenaires au titre de la coopération internationale • Visualisez les Pays Associés 3 Consortium élligibles • ACTIONS INDIRECTES (PROJETS COLLABORATIFS) =FP 7 HEALTH • Au moins trois entités juridiques (définies en tant qu’organisations ou chercheurs individuels comme ci-dessus) doivent participer, chacune d’entre elle devant être établie dans un État membre ou un pays associé, et deux d’entre elles ne pouvant être établies dans le même État membre ou pays associé. Les trois entités juridiques doivent être indépendantes les unes des autres conformément à l’article 6 du RFP, elles ne doivent donc ni être des filiales de la même organisation ni des seconds l’une de l’autre. Doc joint: CP (2 stages) general part 2012 Health-INNOVATION-1

Theme specific information: 1 - Two-stage submission STAGE 1 and STAGE 2. Accessibility to STAGE 2 depends on evaluation o. F STAGE 1 Les soumissions s’effectueront sur ce site: https: //www. epss-fp 7. org/epss/ 2. Two calls: 1 - FP 7 -HEALTH-2013 -INNOVATION-1 as main call with broader topics of which many are tailored for SME participation. 2 - FP 7 -HEALTH-2013 -INNOVATION-2 as a specific call to boost SME participation for innovative solutions in the health sector. 3. Duration of the project must be in line with the realistic planning of the project and so, can be quite short (e. g. 1 -2 years), FP 7 -HEALTH-2013 -INNOVATION-2 call where the maximum project duration is limited to 3 years. Référence Doc joint: CP (2 stages) general part 2012 Health-INNOVATION-1

Theme specific information (1) A –Calendrier: calendrier 2013 non publié Publication of call 20 July 2012 ? Deadline for submission of stage 1 proposals INNOVATION-1: 2 October 2011, ? INNOVATION-2: 25 septembre 2012 ? Evaluation of stage one proposals Decembre 2012 ? Deadline for submission of stage 2 proposals February 2013 ? Coordinators informed of results of stage two proposals April 2013 ? B- Dossier constitué deux parties: « Part A » et « Part B » : Part 1 and Part 2 sont à renseigner a STAGE 1 and STAGE 2 of the proposal Part A : administration details that will be used in the evaluation and further processiong of your proposal. * cf CP(2 stages) Annexes-health 2012 INNOVATION-1 annex 3 page 14 à 23 PART B : detail of your work you attend to carry. * cf Annexes-health 2012 INNOVATION-1 annex 4 page 24 à 46

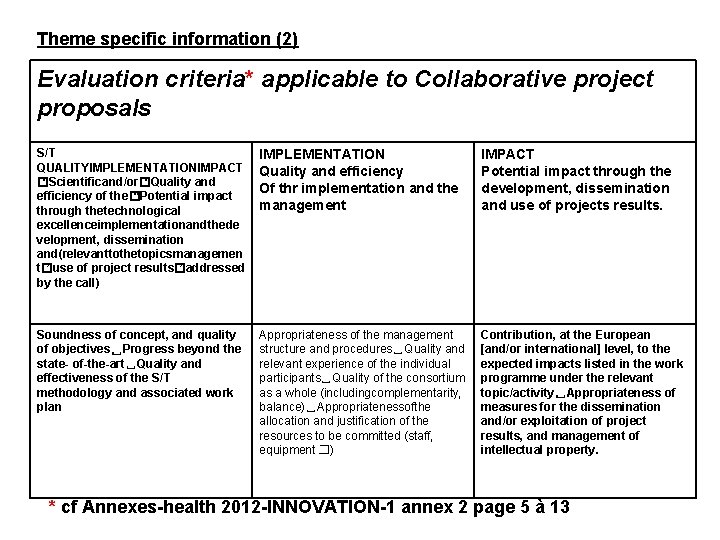
Theme specific information (2) Evaluation criteria* applicable to Collaborative project proposals S/T QUALITYIMPLEMENTATIONIMPACT � “Scientificand/or� “Quality and efficiency of the� “Potential impact through thetechnological excellenceimplementationandthede velopment, dissemination and(relevanttothetopicsmanagemen t� ”use of project results� ”addressed by the call) IMPLEMENTATION Quality and efficiency Of thr implementation and the management IMPACT Potential impact through the development, dissemination and use of projects results. Soundness of concept, and quality of objectives␣Progress beyond the state- of-the-art␣Quality and effectiveness of the S/T methodology and associated work plan Appropriateness of the management structure and procedures␣Quality and relevant experience of the individual participants␣Quality of the consortium as a whole (includingcomplementarity, balance)␣Appropriatenessofthe allocation and justification of the resources to be committed (staff, equipment � …) Contribution, at the European [and/or international] level, to the expected impacts listed in the work programme under the relevant topic/activity␣Appropriateness of measures for the dissemination and/or exploitation of project results, and management of intellectual property. * cf Annexes-health 2012 -INNOVATION-1 annex 2 page 5 à 13

Theme specific information (3) 2 -TOPIQUES* (*fp 7 -health-2013 -orientation-paper-page 9 à 46) : 0. HORIZONTAL TOPICS FOR COLLABORATIVE PROJECTS RELEVANT FOR THE WHOLE OF THEME HEALTH - FP 7 -HEALTH-2013 -INNOVATION-2. : Boosting the translation of FP projects' results into innovative applications for health. 1. BIOTECHNOLOGY, GENERIC TOOLS AND MEDICAL TECHNOLOGIES FOR HUMAN HEALTH -HEALTH. 2013. 1. 2 -1: Development of imaging technologies for therapeutic interventions in rare diseases. . -HEALTH. 2013. 1. 3 -1: Modelling toxic responses in case studies for predictive human • safety assessment. . -HEALTH. 2013. 1. 3 -2: Innovative approaches to address adverse immune reactions to biomedical devices, implants and transplant tissues-HEALTH. 2013. 1. 3 -3: Safety and efficacy of therapeutic vaccines -HEALTH. 2013. 1. 3 -4: Development of alternative in vitro, analytical, immunochemical, and other test methods for quality control of vaccines. 1. 4 stem cell research: HEALTH. 2013. 1. 4 -1. Controlling differentiation and proliferation in human stem cells intended for therapeutic use.

2. TRANSLATING RESEARCH FOR HUMAN HEALTH: � 2. 1 INTEGRATING BIOLOGICAL DATA AND PROCESSES: LARGE-SCALE DATA GATHERING, SYSTEMS BIOLOGY - HEALTH. 2013. 2. 1. 1 -1: Functional validation in animal and cellular models of genetic determinants of diseases and ageing processes. - HEALTH. 2013. 2. 1. 1 -2: High impact research initiative on metagenomics for personalised medicine approaches 2. 2 RESEARCH ON THE BRAIN AND RELATED DISEASES, HUMAN DEVELOPMENT AND AGEING - HEALTH. 2013. 2. 2. 1 -1: Prospective longitudinal data collection and Comparative Effectiveness Research (CER) for traumatic brain injury (TBI). -HEALTH. 2013. 2. 2. 1 -2: Development of effective imaging tools for diagnosis, monitoring and management of mental disorders 28. --HEALTH. 2013. 2. 2. 1 -3: Paediatric conduct disorders characterised by aggressive traits and/or social impairment: from preclinical research to treatment. --HEALTH. 2013. 2. 2. 1 -4: Patho-physiology and therapy of epilepsy and epileptiform disorders. --HEAL TH. 2013. 2. 2. 1 -5: Understanding and controllingpain.

2. 3 TRANSLATIONAL RESEARCH IN MAJOR INFECTIOUS DISEASES: TO CONFRONT MAJORTHREATS TO PUBLIC HEALTH. -HEALTH. 2013. 2. 3. 0 -1: Innovation in vaccines. . -HEALTH. 2013. 2. 3. 1 -1: Drugs and vaccines for infections that have developed or are at the risk of developing significant anti-microbial resistance. -HEALTH. 2013. 2. 3. 1 -2: Stratified approaches to antibacterial and/or antifungal treatment. -HEALTH. 2013. 2. 3. 3 -1: Clinical management of patients in severe epidemics. -HEALTH. 2013. 2. 3. 4 -1: Neglected infectious diseases of Central and Eastern Europe. - HEALTH. 2013. 2. 3. 4 -2: Drug development for neglected parasitic diseases. FP 7 -

2. 4 TRANSLATIONAL RESEARCH IN OTHER MAJOR DISEASES HEALTH. 2013. 2. 4. 1 -1: Investigator-driven treatment trials to combat or prevent metastases in patients with solid cancer. HEALTH. 2013. 2. 4. 1 -2: Strengthening the cancer patient's immune system. HEALTH. 2013. 2. 4. 1 -3: Investigator-driven supportive and palliative care clinical trials andobservationalstudies. 2. 4. 2 Cardiovascular diseases. HEALTH. 2013. 2. 4. 2 -1: Discovery research to reveal novel targets for cardiovascular diseasetreatment. HEALTH. 2013. 2. 4. 2 -2: Comparative effectiveness research of existing technologies for prevention, diagnosis and treatment of cardiovascular diseases. HEALTH. 2013. 2. 4. 2 -3: Optimising lifestyle interactions in the prevention and treatment of cardiovascular disease across the lifespan. 2. 4. 3 Diabetes and obesity: Closed 20132. 4. 4 Rare diseases: Closed 20132. 4. 5 Other chronic diseases: Closed 2013

3. OPTIMISING THE DELIVERY OF HEALTHCARE TO EUROPEAN CITIZENS -HEALTH. 2013. 3. 1 -1: Comparative effectiveness research (CER) in health systems and health services interventions. -HEAL TH. 2013. 3. 3 -1: Social innovation 3 fo rhealth promotion. 4. OTHER ACTIONS ACROSS THE HEALTH THEME -HEALTH. 2013. 4. 1 -1: Supporting industrial participation in EU-funded research in the Health sector. -HEALTH. 2013. 4. 1 -2: Interactions between EU legislation and health research and/or innovation and the effects of its application and implementation on health research and/or innovation. -HEALTH. 2013. 4. 1 -3: Support for Presidency events: Organisation of supporting actions and events associated to the Presidency of the European Union. - HEALTH. 2013. 4. 1 -4: Preparing the future for health research and innovation. -FP 7 -HEALTH. 2013. 4. 1 -5: Global initiative on gene-environment interactions in diabetes/obesityinspecificpopulations. -FP 7 -HEAL TH. 2013. 4. 1 -6: Mapping chronic non-communicable diseases research activities.

4. 2 RESPONDING TO EU POLICY NEEDS: -HEALTH. 2013. 4. 2 -1: Investigator-driven clinical trials for off-patent medicines using innovative, age-appropriate formulations and/or delivery systems. -HEALTH. 2013. 4. 2 -2: Adverse drug reaction research. . -HEALTH. 2013. 4. 2 -3: New methodologies for clinical trials for small population groups

Others mains points of the projects: -Ethical issues: It is particularly important that applicants address the potential ethical issues of their proposals, both in the proposed methodology and the possible implications of the results. -Use of animals in research: protocole on the protection and welfare of animals -Gender dimension: all projetc are encouraged to have balanced participation of women and men - Socio-economic dimension of research: account should be taken of possible socio-economic impacts of research, including its intended and unintended consequences and the inherent risks and opportunities. - • Statistics in health research: the proposal must include and explain the statical aspect. - Funding schemes cf doc joint Voir référence. doc ci joint « FP 7 -health-orientation paper. Page 7 et 8

• LES ETAPES DU MONTAGE DU PROJET: • 1 - identifier le sujet de la recherche. Voir les questions à se poser dans ref jointe « le petit guide d’aide au montage de projets FP 7_CLORA » 2 - s’assurer de la conformité du sujet par rapport aux topiques proposés dans le FP 7 Health (cf liste des topiques jointes et détails des conditions de l’AO en fp 7 -health-2013 -orientation -paper-120402). Pour cette étape nous pouvons proposer votre aide avec le PCN ( Point de contact Nationaux) monsieur Nacer Boubenna INSERM, coordinateur du PCN. Étape clé+++ • • • 3 - Identifier les partenaires du consortium du projet: liens utiles http: //www. fitforhealth. eu/-Cordis général, réseau OSEO pour la recherche de PME pour le consortium https: //cordis. europa. eu/partners/web/guest/home 4 - Identifier les étapes du projets TRES PRECISEMENT cad déroulement dans le temps, faisabilité, description des tâches de chacun des membres du consortium. Ref: CP (2 stages) general part 2012 Health-INNOVATION-1 CP (2 stages) Annexes-health 2012 -INNOVATION-1 5 - Ecriture du projet (exigence éthique , de genre, de méthodo et statistique. . ). Coordinations Inserm il y a possibilité d'être accompagnés (cf: http: //dircom. inserm. fr/europe/linserm-vousaccompagne-d. html)

DATE DE REUNION et LIENS UTILES Journée d’information sur tous les prochains appels d’offres européens aura lieu le 15 juin prochain à l’hôpital Cochin. Pôle Europe DRCD-PCN INSERM (suivra planning et programme) - CORDIS : http: //cordis. europa. eu/fp 7/health/ - Point de contact national santé PCN (France) comportant les dernières informations utiles http: //www. eurosfaire. prd. fr/7 pc/health/ Nacer BOUBENNA Inserm +33 1 44 23 61 90 - Lien du site Fitforhealth via le réseau OSEO pour la recherche de PME pour le consortium : http: //www. fitforhealth. eu/participate. aspx; contact : mathilde. picco@oseo. fr - Alix Pillot Pôle Europe (DRCD)-Assistance Publique-Hôpitaux de Paris 10 Bureau : +33 (0)1 40 27 46 13 Fax : +33 (0)1 44 84 17 88
 Expertise france appel à projet
Expertise france appel à projet Cadre commun europeen reference pour langues
Cadre commun europeen reference pour langues Institut universitaire européen de la mer
Institut universitaire européen de la mer Serpent monétaire européen
Serpent monétaire européen Radical vs disruptive innovation
Radical vs disruptive innovation Mysite socccd
Mysite socccd Formule de politesse
Formule de politesse Albert de munster chanson
Albert de munster chanson Croc telephone
Croc telephone Procès d'intention sophisme
Procès d'intention sophisme Plan d appel téléphonique croc
Plan d appel téléphonique croc Mascnss
Mascnss Chant quand le ciel est bleu mon garçon
Chant quand le ciel est bleu mon garçon Ethos pathos og logos
Ethos pathos og logos Logos pathos ethos
Logos pathos ethos Différence entre appel et vocation
Différence entre appel et vocation Programme ce soir la tlvision
Programme ce soir la tlvision Dubbufet
Dubbufet