AP LAB 12 DISSOLVED OXYGEN Introduction to Water

AP LAB #12 DISSOLVED OXYGEN Introduction to Water Quality & AP Lab #12 Ms. Gaynor AP Biology

Why is Water SO Important? ¡It is a renewable resource that is becoming polluted & destroyed l. Recycles cycle” ¡WATER through “water (hydrologic) = SURVIVAL ¡~ 80% of body = water ¡Only 1% of water on Earth = fresh ¡Used for domestic, industrial, commercial, & recreational use

Why Test Water Quality? ¡ To monitor human and ecological impact and its affect on aquatic life/ aquatic habitats l Monitoring means “to collect data” on water to access affects of pollutants ¡ Water quality can disrupt water chemistry harmful to aquatic food chain

AP LAB #12: Dissolved Oxygen (DO) = the amount of oxygen gas dissolved in water l 95% more O 2 in air than in cold water ¡ [DO] ¡ l l ¡ used to determine whether the biological activities requiring O 2 are occurring ALSO an indicator of pollution O 2 important in metabolic processes indicator of water quality

Why is DO important? Oxygen must be in water in a free state (O 2) before organisms can use it l This depends on physical and chemical properties of water. ¡ Sewage and detritus (dead organic matter) depletes DO b/c it requires a lot of O 2 as it decomposes ¡

How does the O 2 get into the water? 1. 2. 3. 4. primary productivity (photosynthesis) Atmosphere (air) Winds Mixing (waves, currents etc…)
![6 MAIN Factors that affect [DO] 1. Air pressure above water affects [DO] l 6 MAIN Factors that affect [DO] 1. Air pressure above water affects [DO] l](http://slidetodoc.com/presentation_image_h2/6b06b2910c228b3feda2ba6652742243/image-7.jpg)
6 MAIN Factors that affect [DO] 1. Air pressure above water affects [DO] l Less O 2 present at higher elevations (b/c air is less dense) l water at higher elevations contain less O 2
![6 MAIN Factors that affect [DO] (con’t) 2. Photosynthesis (adds O 2) & cell 6 MAIN Factors that affect [DO] (con’t) 2. Photosynthesis (adds O 2) & cell](http://slidetodoc.com/presentation_image_h2/6b06b2910c228b3feda2ba6652742243/image-8.jpg)
6 MAIN Factors that affect [DO] (con’t) 2. Photosynthesis (adds O 2) & cell aerobic respiration (uses/takes away O 2) ¡Photosynthesis l in bright light aquatic producers make more O 2 ¡Cellular Respiration (metabolic) activity l aquatic organisms use more O 2
![6 MAIN Factors that affect [DO] (con’t) 3. As salinity and temperature of water 6 MAIN Factors that affect [DO] (con’t) 3. As salinity and temperature of water](http://slidetodoc.com/presentation_image_h2/6b06b2910c228b3feda2ba6652742243/image-9.jpg)
6 MAIN Factors that affect [DO] (con’t) 3. As salinity and temperature of water increases [DO] decreases l Salinity = amount of salts dissolved in water l More Salts= less [DO] l Less Salts= more [DO] l Temperature l Warmer H 2 O = low [DO] l Cold H 2 O = higher [DO]
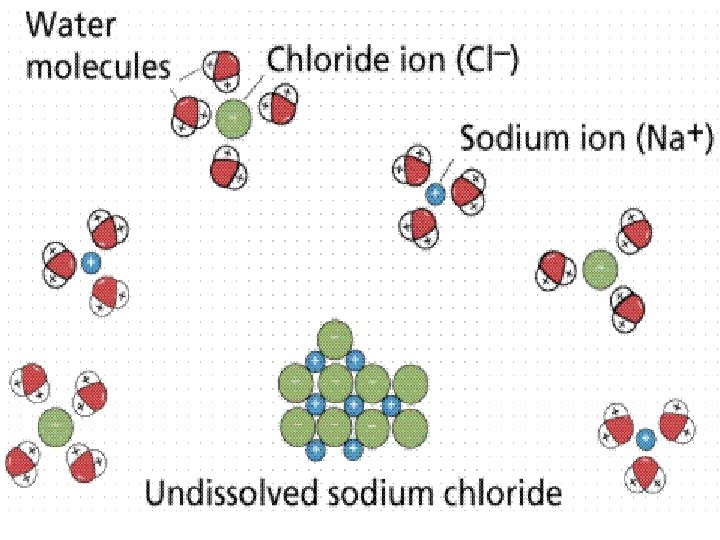
![6 MAIN Factors that affect [DO] (con’t) 4. Decomposition activity l As organic (DEAD) 6 MAIN Factors that affect [DO] (con’t) 4. Decomposition activity l As organic (DEAD)](http://slidetodoc.com/presentation_image_h2/6b06b2910c228b3feda2ba6652742243/image-11.jpg)
6 MAIN Factors that affect [DO] (con’t) 4. Decomposition activity l As organic (DEAD) material decays, microorganisms that decompose material consume oxygen (O 2) 5. Mixing and turbulence l Wave action, waterfalls, and rapids all aerate (add “air” to) water and increase the O 2 concentration.
![6 MAIN Factors that affect [DO] (con’t) 6. Wind l. Windless nights = low(er) 6 MAIN Factors that affect [DO] (con’t) 6. Wind l. Windless nights = low(er)](http://slidetodoc.com/presentation_image_h2/6b06b2910c228b3feda2ba6652742243/image-12.jpg)
6 MAIN Factors that affect [DO] (con’t) 6. Wind l. Windless nights = low(er) [DO] l. High winds = High [DO]
![Where is the greatest [DO]? lowest [DO]? Where is the greatest [DO]? lowest [DO]?](http://slidetodoc.com/presentation_image_h2/6b06b2910c228b3feda2ba6652742243/image-13.jpg)
Where is the greatest [DO]? lowest [DO]?

DO and Trophic States ¡ 2 classifications ¡ Eutrophic = “well nourished” high nutrient [ ] lots of plant life as well as respiration (O 2 fluctuates) l GREENER in color (from algae) Oligotrophic = low nutrient [ ] low plant growth (always rich in O 2 ) l CLEAR in color, very “blue” l Think: “Ewww” a green lake ¡ Think: “Ohhh” a nice clear blue lake

EUTROPHIC OLIGOTROPHIC
Lab #12: Dissolved Oxygen ¡Concepts l dissolved O 2 (DO) l primary productivity (aka-photosynthesis) ¡measured in 3 ways: l amount of CO 2 used l rate of glucose/ sugar (biomass) formation l rate of O 2 production l net productivity vs. gross productivity l cellular respiration
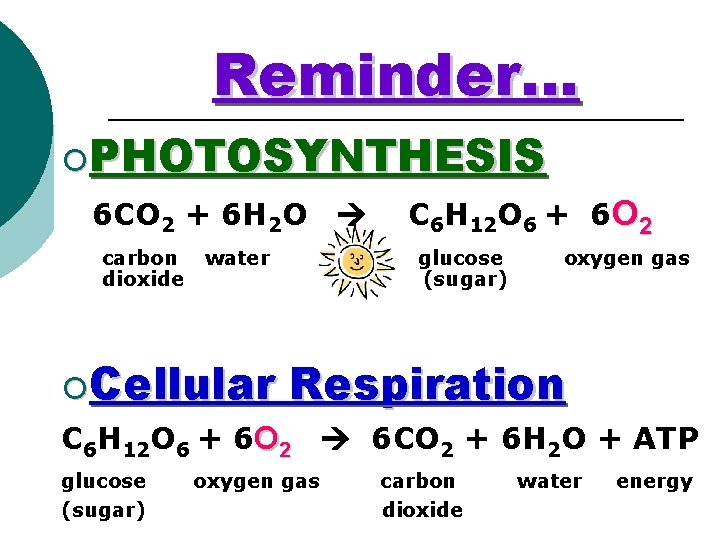
Reminder… ¡PHOTOSYNTHESIS 6 CO 2 + 6 H 2 O carbon dioxide water ¡Cellular C 6 H 12 O 6 + 6 O 2 glucose (sugar) oxygen gas Respiration C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O + ATP glucose (sugar) oxygen gas carbon dioxide water energy
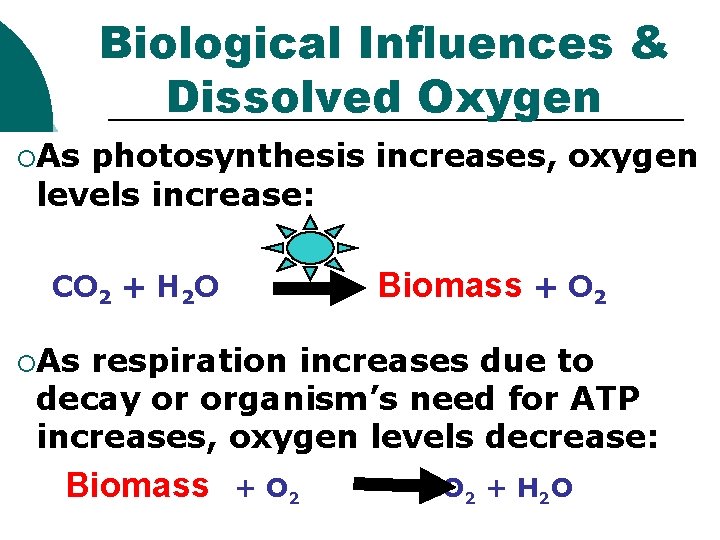
Biological Influences & Dissolved Oxygen ¡As photosynthesis increases, oxygen levels increase: Biomass + O 2 CO 2 + H 2 O ¡As respiration increases due to decay or organism’s need for ATP increases, oxygen levels decrease: Biomass + O 2 CO 2 + H 2 O

Productivity in a Aquatic Environment ¡ Primary productivity = rate at which organic (carbon based) materials are stored through photosynthesis l Can be measured in three ways: 1. The amt of carbon dioxide used 2. The rate of sugar formation 3. The rate of oxygen production
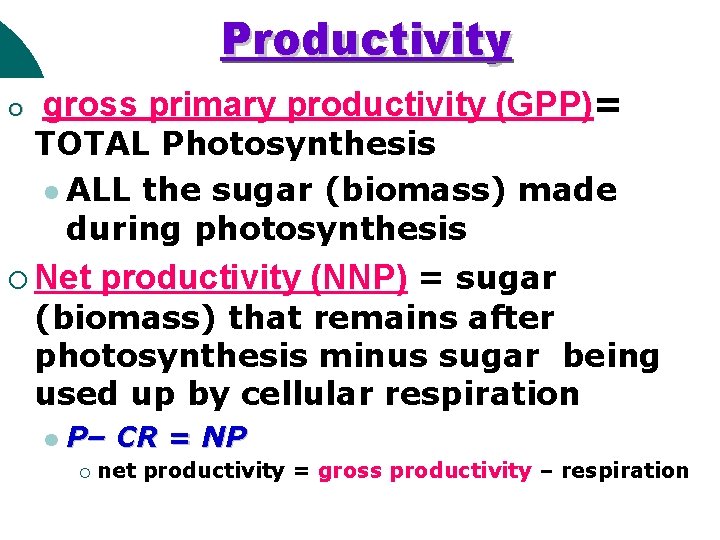
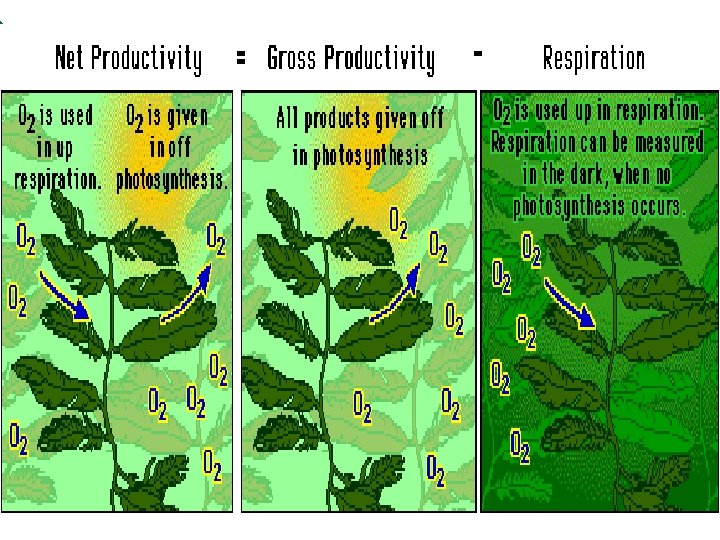
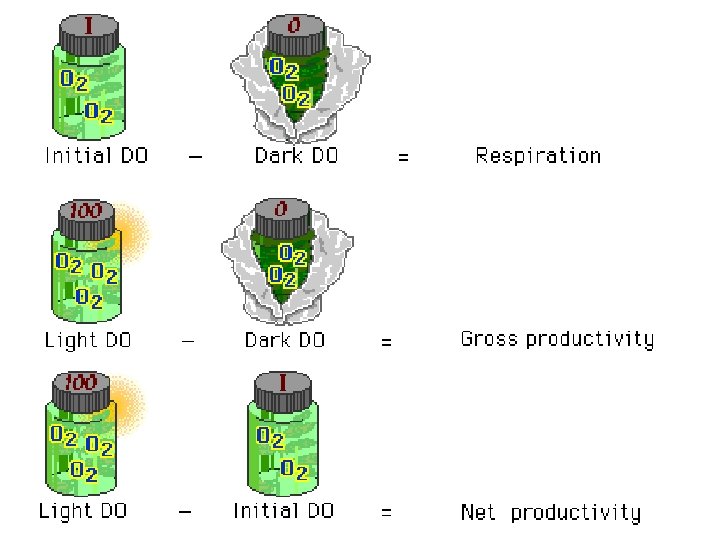
Productivity ¡ gross primary productivity (GPP)= TOTAL Photosynthesis l ALL the sugar (biomass) made during photosynthesis ¡ Net productivity (NNP) = sugar (biomass) that remains after photosynthesis minus sugar being used up by cellular respiration l P– CR = NP ¡ net productivity = gross productivity – respiration

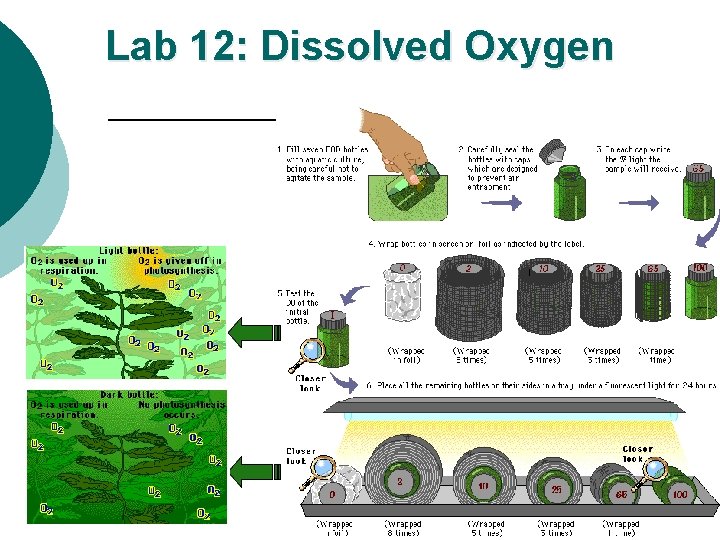
Light & Dark Bottle Method Light bottle represent daytime ¡ Dark bottle can represent night time ¡ BOTTLE #1 (5 light bottles 1 light control bottle) **exposed to light **Acts as initial bottle as well BOTTLE #2 (1 dark bottle) **NOT exposed to light
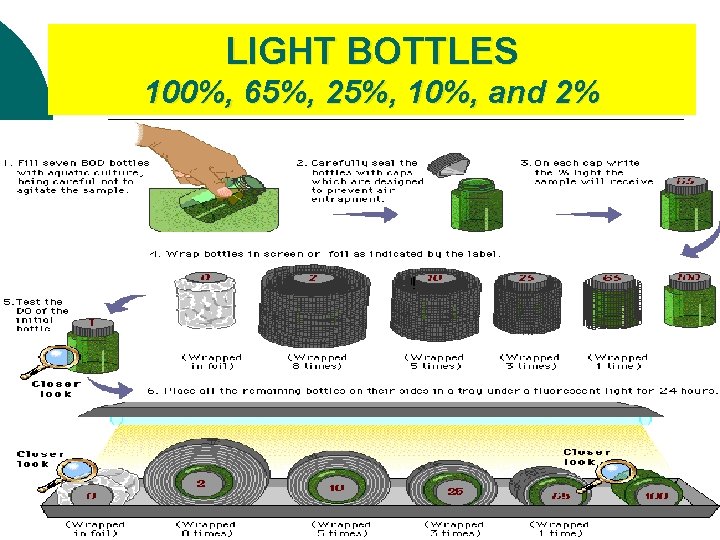
LIGHT BOTTLES 100%, 65%, 25%, 10%, and 2% 2004 -2005
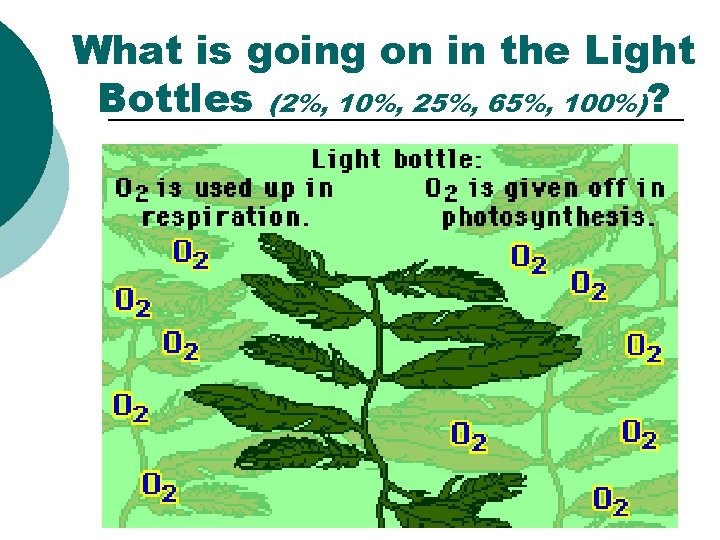
What is going on in the Light Bottles (2%, 10%, 25%, 65%, 100%)?
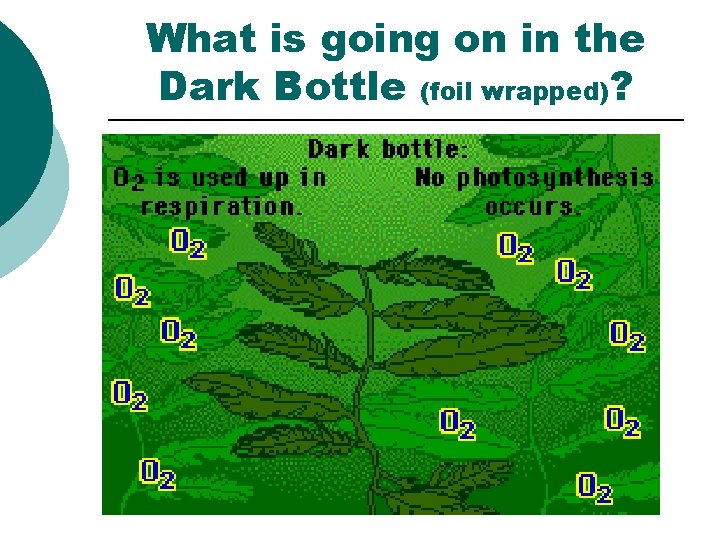
What is going on in the Dark Bottle (foil wrapped)? ¡
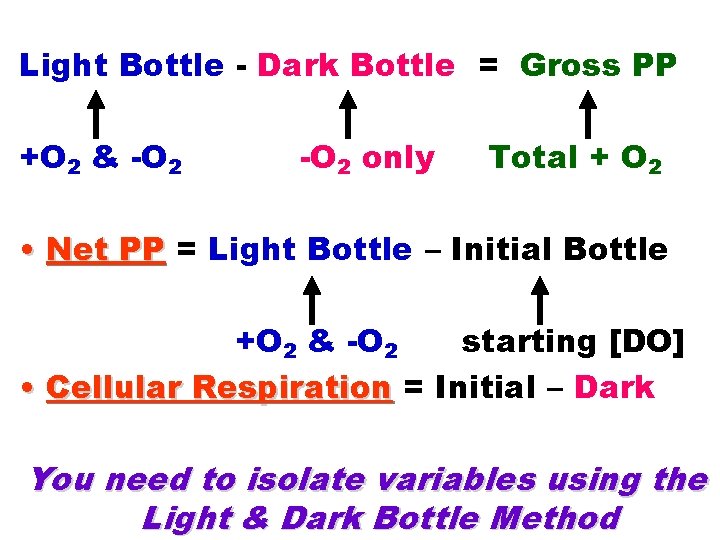
Light Bottle - Dark Bottle = Gross PP +O 2 & -O 2 only Total + O 2 • Net PP = Light Bottle – Initial Bottle +O 2 & -O 2 starting [DO] • Cellular Respiration = Initial – Dark You need to isolate variables using the Light & Dark Bottle Method

Lab #12: Experimental Design (setup) • This lab has 2 PARTS and will take 2 days!
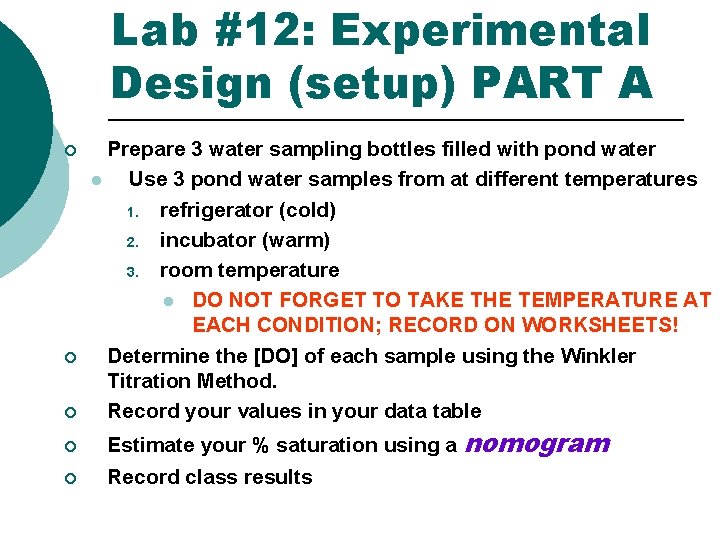
Lab #12: Experimental Design (setup) PART A ¡ ¡ ¡ Prepare 3 water sampling bottles filled with pond water l Use 3 pond water samples from at different temperatures 1. refrigerator (cold) 2. incubator (warm) 3. room temperature l DO NOT FORGET TO TAKE THE TEMPERATURE AT EACH CONDITION; RECORD ON WORKSHEETS! Determine the [DO] of each sample using the Winkler Titration Method. Record your values in your data table ¡ Estimate your % saturation using a ¡ Record class results nomogram
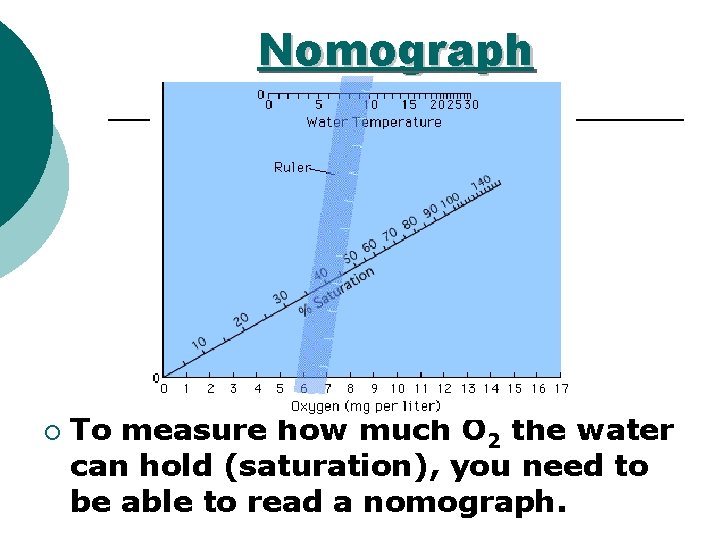
Nomograph ¡ To measure how much O 2 the water can hold (saturation), you need to be able to read a nomograph.

Lab #12: Experimental Design (setup) PART B ¡ ¡ ¡ ¡ Fill 7 water sampling bottles with pond water (BOD bottles for “biological oxygen demand”) Add piece of plant to each bottle. Make sure that they are the same! Count leaves, measure, etc. CONTROL THE VARIABLES! Label bottles-I, D, 100%, 65%, 25%, 10%, and 2% Determine the DO for the initial bottle using Winkler Titration method. l THIS IS YOUR BASE LINE (CONTROL GROUP); the amount of DO ALL your samples start with! Cover D (dark) bottle with aluminum foil Cover the remaining bottles with screens (see procedure for details) Place bottles under the light source. Wait 24 hours, then measure DO levels of remaining samples using Winkler Titration method.
Lab 12: Dissolved Oxygen 2004 -2005

Hints for Setting Up Lab #12 ¡ Rinse sampling bottle 3 x with sample water ¡ Submerge bottle in water; allow to fill. ¡ Tap bottle to release air bubbles l You do NOT want extra atmospheric O 2 ¡ While bottle is submerged, replace cap l If there air bubbles in the bottle, empty and repeat ¡ Preserve sample immediately. Test within 2 hours.

More Hints for Setting Up Lab #12 Do NOT leave water samples uncapped! Hello O 2 will get in!!! ¡ Use rubber bands to secure the screens ¡ NOTE: We are using HORNWORT plant not Anacharis ¡ l Please FIX on your worksheets!

Winkler Titration Method (PART 1) How Do You FIX Samples Correctly? To “fix” your samples, follow for each sample bottle: (“fix” = trap DO in sample) 1. Uncap bottle 2. Add 8 drops of Mg. SO 4 (manganous sulfate) to bottle (can cause cancer/stain) 3. Add 8 drops of alkaline potassium iodide azide to bottle (can cause cancer/stain) 4. Cap bottles and mix. A precipitate will form! Allow precipitate to settle to shoulder of bottle. 5. Add a spoonful (1 g) of sulfamic acid powder to bottle (STRONG ACID) 6. Cap and mix. Precipitate should dissolve!

Winkler Titration Method (PART 2) How Do You Determine the amount of DO in the samples Correctly? To determine the [DO] of your samples, follow for each sample bottle, including the initial bottle: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. Uncap bottle Carefully fill the titration tube/cup to the 20 m. L line. Fill the titration syringe to the “ 0” (zero) line with sodium thiosulfate. Add 1 drop AT A TIME to sample and swirl after each drop. Do this until you get a FAINT yellow color. Remove titration syringe Add 8 drops of starch indicator solution Swirl the sample. The sample should now be BLUE! No blue = no measurable [DO] or too much sodium thiosulfate Add sodium thiosulfate 1 drop at a time and SWIRL until BLUE color disappears. If you finish the syringe and it is still blue, fill syringe again and continue. Read titration syringe scale for result in mg DO/liter Fill in data table; multiple DO in mg/L by 0. 698 to convert to m. L/L
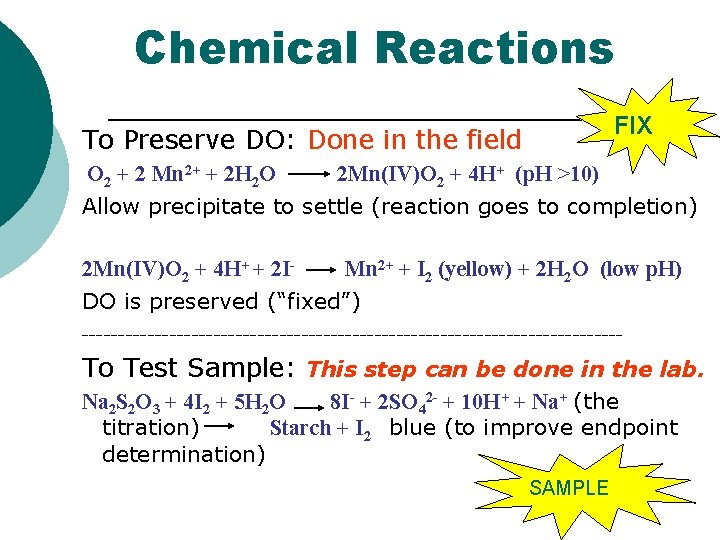
Chemical Reactions FIX To Preserve DO: Done in the field O 2 + 2 Mn 2+ + 2 H 2 O 2 Mn(IV)O 2 + 4 H+ (p. H >10) Allow precipitate to settle (reaction goes to completion) 2 Mn(IV)O 2 + 4 H+ + 2 IMn 2+ + I 2 (yellow) + 2 H 2 O (low p. H) DO is preserved (“fixed”) ------------------------------------- To Test Sample: This step can be done in the lab. Na 2 S 2 O 3 + 4 I 2 + 5 H 2 O 8 I- + 2 SO 42 - + 10 H+ + Na+ (the titration) Starch + I 2 blue (to improve endpoint determination) SAMPLE
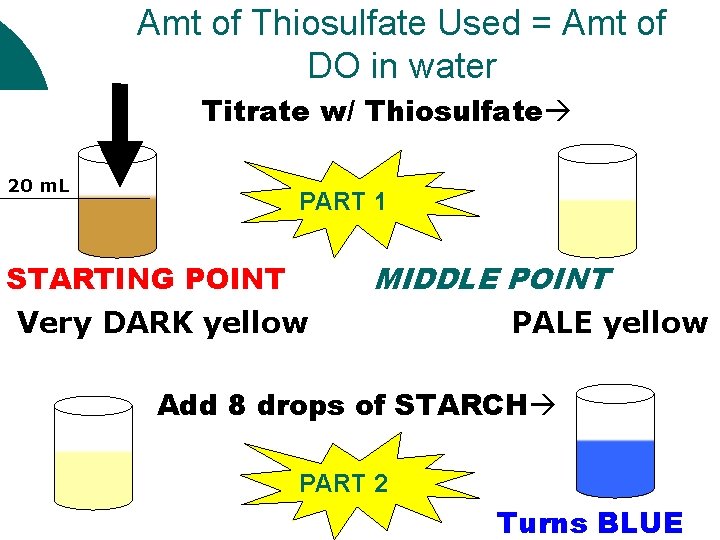
Amt of Thiosulfate Used = Amt of DO in water Titrate w/ Thiosulfate 20 m. L PART 1 STARTING POINT Very DARK yellow MIDDLE POINT PALE yellow Add 8 drops of STARCH PART 2 Turns BLUE
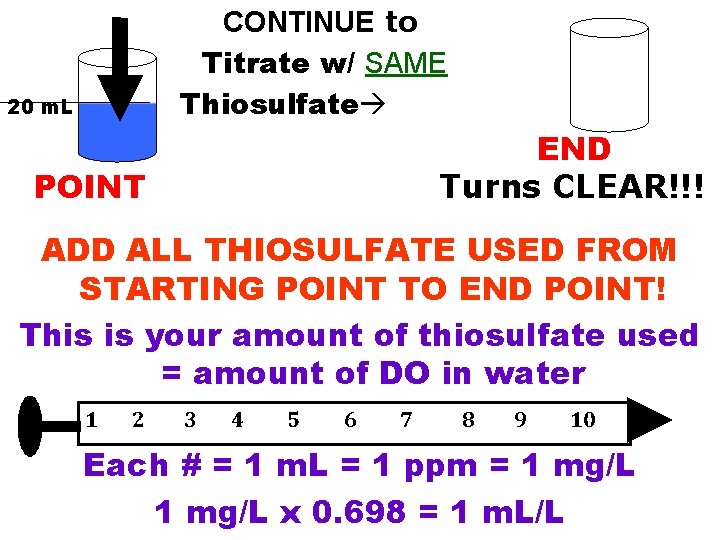
CONTINUE to Titrate w/ SAME Thiosulfate 20 m. L END Turns CLEAR!!! POINT ADD ALL THIOSULFATE USED FROM STARTING POINT TO END POINT! This is your amount of thiosulfate used = amount of DO in water 1 2 3 4 5 6 7 8 9 10 Each # = 1 m. L = 1 ppm = 1 mg/L x 0. 698 = 1 m. L/L


Dissolved Oxygen ¡Can be measured in absolutes (how much O 2 is actually in the water) or by % saturation ¡% saturation - measure of DO compared to how much DO COULD be in the water l Water at a higher temperature CAN NOT hold as much oxygen as cold water (use nonmaogram)

Lab 12: Dissolved Oxygen ¡Conclusions to Think About… l temperature = dissolved O 2 l light = photosynthesis = O 2 production l. O 2 is consumed during cellular respiration l respiration = dissolved O 2 (consumption of O 2)
- Slides: 42