AP CHEMISTRY TUTORIALS 02 CHP03 BALANCING EQUATIONS Srinivasan
AP CHEMISTRY TUTORIALS– 02 CHP-03: BALANCING EQUATIONS Srinivasan G. Srivilliputhur December 10, 2015 Must know formulas, ionic charges, oxidations states. .

Today’s Overview 1 • Naming Polyatomic Ions 2 • Net Ionic Equations 3 • AP Examples 4 • Review: Experimental Methods
Next Tutorial 1 • Balance Redox Equations 2 • Jeopardy Game: Name Polyatomic Compounds

Ion Formation & The Periodic Table +/4 TRANSITION METALS FORM VARIABLE CHARGE IONS

1 • Oxidation States: Review

Oxidation States (OS) • Help us describe and balance redox reactions • Are imaginary charges assigned to elements in a compound -

Oxidation States (OS) -

1 • Naming Polyatomic Ions


Naming Some Polyatomic Ions • Mnemonic • NICK the CAMEL ate a CLAM for SUPPER • in PHOENIX NICK = NO • #Consonants = O = 3 • #Vowels = Charge = 1 - Take only words with >= 4 letters First letter tells us the element before oxygen Count number of consonants and vowels in each word ü Number of Oxygen = consonant count ü Polyatomic ion charge = vowel count • Nitrate = NO– 3 CAMEL = CO • #Consonants = O = 3 • #Vowels = Charge = 2 - CLAM = Cl. O • #Consonants = O = 3 • #Vowels = Charge = 1 - • Carbonate = CO 2– 3 • Chlorate =- Cl. O– 3

Deriving Other Polyatomic Ions -
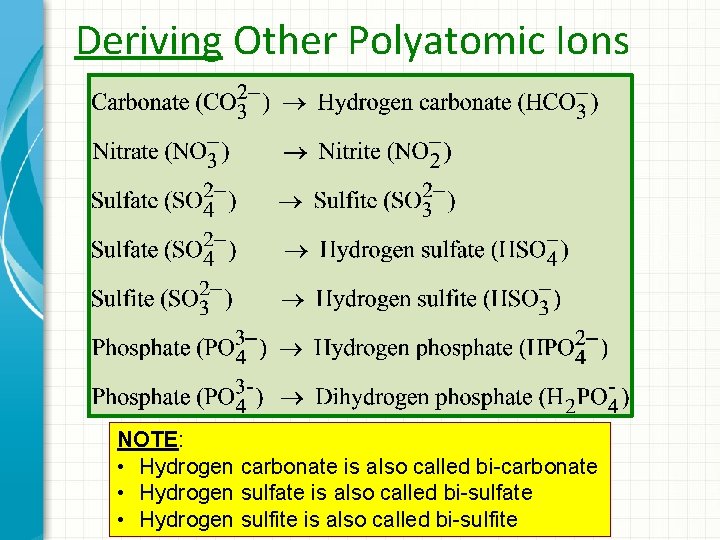
Deriving Other Polyatomic Ions NOTE: • Hydrogen carbonate is also called bi-carbonate • Hydrogen sulfate is also called bi-sulfate • Hydrogen sulfite is also called bi-sulfite

MEMORIZE Other Polyatomic Ions -

2 • Net Ionic Equations

Net Ionic Equations in Six Easy Steps 1. 2. 3. 4. 5. 6. Read the Question, circle states of reactants/products, and Identify Keywords* Identify Reaction Type Predict Products Check Solubility (Memorize the Rules) Write Ionic Equation Remove Spectator Ions net ionic equation Remember H 2 O as H–––OH

Keywords (partial list) • • • Acidified solution (added H+ ions in solution); Alkaline solution (added OH- ions in solution); Concentrated; dilute; solution; weak acid/base Equimolar solution; Excess (= not a limiting reagent) Completely (maybe a limiting reagent) Mixed (= reacted); Oxidize(= reacted) Solid; crystals; powder; precipitate; strip; filings; Bubbled; gas; Catalyst (= not consumed in the reaction);

The Steps in a Template e p y T Predict Products Check Solubility Net Ionic Equation Keywords

Solubility Rules

Exercises 1) Dilute sulfuric acid is added to a solution of barium acetate 2) Gaseous hydrofluoric acid reacts with solid silicon dioxide 3) Sodium hydrogen carbonate is dissolved in water 4) Solid ammonium carbonate is heated 5) Calcium nitrate solution is reacted with concentrated potassium fluoride 6) Potassium hydroxide solution is reacted with acetic acid

3 • AP Chemistry Example

2008 AP Chemistry: (Q-2) Answer the following questions relating to gravimetric analysis. In the first of two experiments, a student is assigned the task of determining the moles of water in one mole of Mg. Cl 2⋅n. H 2 O. The student collects the data shown in the following table.

2008 AP Chemistry: (Q-2) (a) Explain why the student can correctly conclude that the hydrate was heated a sufficient number of times in the experiment. Negligible mass loss during the third heating indicates that all the water of hydration was likely driven off.

2008 AP Chemistry: (Q-2) b) (i) calculate the total number of moles of water lost when the sample was heated, and H 2 O lost = 25. 825 − 23. 977 = 1. 848 g Loss = 1. 848 g/18. 02 g/mol = 0. 1026 mol H 2 O

2008 AP Chemistry: (Q-2) b) (ii) determine the formula of the hydrated compound. • mass of anhydrous Mg. Cl 2 = 23. 977 − 22. 347 = 1. 630 g • 1. 630 g/95. 20 g/mol = 0. 01712 mol Mg. Cl 2 • 0. 1026 mol H 2 O/0. 01712 mol Mg. Cl 2 ~ 6 moles of H 2 O per mole of Mg. Cl 2 Formula: Mg. Cl 2⋅6 H 2 O

2008 AP Chemistry: (Q-2) c) A different student heats the hydrate in an uncovered crucible, and some of the solid spatters out of the crucible. This spattering will have what effect on the calculated mass of the water lost by the hydrate? Justify your answer. The mass of the solid that was lost in splatter will be assumed to be water when it also included some Mg. Cl 2. Thus, the calculated moles of water will be wrong

2008 AP Chemistry: (Q-2) In the second experiment, a student is given 2. 94 g of a mixture containing anhydrous Mg. Cl 2 and KNO 3. To determine the percentage by mass of Mg. Cl 2 in the mixture, the student uses excess Ag. NO 3(aq) to precipitate the chloride ion as Ag. Cl(s). d) Starting with the 2. 94 g sample of the mixture dissolved in water, briefly describe the steps necessary to quantitatively determine the mass of the Ag. Cl precipitate. • Filter the Ag. Cl precipitate • Wash and dry the precipitate completely • Determine the mass of Ag. Cl • 2 points for all the steps • 1 point for any 2 steps

2008 AP Chemistry: (Q-2) In the second experiment, a student is given 2. 94 g of a mixture containing anhydrous Mg. Cl 2 and KNO 3. To determine the percentage by mass of Mg. Cl 2 in the mixture, the student uses excess Ag. NO 3(aq) to precipitate the chloride ion as Ag. Cl(s). e) The student determines the mass of the Ag. Cl precipitate to be 5. 48 g. On the basis of this information, calculate each of the following: (i) The number of moles of Mg. Cl 2 in the original mixture • 5. 48 g Ag. Cl/143. 32 g/mol = 0. 0382 mol Ag. Cl • 0. 0382 mol Ag. Cl x (1 mol Cl/1 mol Ag. Cl) x (1 mol Mg. Cl 2/2 mol Cl) = 0. 0191 mol Mg. Cl 2

2008 AP Chemistry: (Q-2) In the second experiment, a student is given 2. 94 g of a mixture containing anhydrous Mg. Cl 2 and KNO 3. To determine the percentage by mass of Mg. Cl 2 in the mixture, the student uses excess Ag. NO 3(aq) to precipitate the chloride ion as Ag. Cl(s). e) The student determines the mass of the Ag. Cl precipitate to be 5. 48 g. On the basis of this information, calculate each of the following: (ii) The percent by mass of Mg. Cl 2 in the original mixture • 0. 0191 mol Mg. Cl 2 95. 20 g/mol Mg. Cl 2 = 1. 82 g Mg. Cl 2 • (1. 82 g Mg. Cl 2/2. 94 g sample) x 100 = 61. 9% Mg. Cl 2 by mass

4 • Separating Mixtures: Review

Filtration: Immiscible substances • Also used to separate precipitates from liquid

Flotation: Modified Filtration Skim the froth (e. g. chocolate floating)

Evaporation: E. g. Salt in Water • • Salt Solution Distillate (water) Separate dissolved substance from solvent Evaporate the liquid to separate

Distillation: E. g. Salt in Water • • • (Fractional) distillation is best to separate a solution of miscible liquids (liquids that dissolve in each other) Principle: Uses the differences in boiling points of liquids The column has beads to help the rising gas to slowly condense and re-evaporate many times, before collecting distillate

5 • Tutorials: Winter Break • If you wish, we can continue tutorials during the winter break (at one of the Denton Public Library Branches if room is available) • Create a email list – Kesavan will coordinate • Plan Thermochemistry Chapters 6 and 17 Electrochemistry Chapter 18
- Slides: 34