AP Chemistry Kinetics AP Chemistry Kinetics The study







![AP Chemistry Concentration and Rate • This means Rate [NH 4+] − Rate [NO AP Chemistry Concentration and Rate • This means Rate [NH 4+] − Rate [NO](https://slidetodoc.com/presentation_image_h2/6629d4963cb673a62499d3c5c0aa1eae/image-28.jpg)









- Slides: 62

AP Chemistry Kinetics

AP Chemistry Kinetics The study of the speeds, or rates, of chemical reactions. Goals: 1. Understand how to determine the rates at which reactions occur 2. Understand the factors that control these rates.

AP Chemistry Because reactions involve the breaking and forming of bonds, the speed depends on the nature of the reactants. It’s about the frequency of collisions between molecules. More collisions = faster reaction

AP Chemistry I feel the need – the need for speed! -Maverick “Top Gun” 1. 2. 3. 4. Factors that affect Reaction Rates The physical state of the reactants. Reactants in the same state are more likely to react. Reactants in different phases are limited to the area of contact (ie surface area) Concentration of the reactants. High concentration of one reactant makes reactions easier. The temperature at which the reaction occurs. (“heat ‘em up, speed ‘em up!) – gases at a high temperature have a higher kinetic energy and therefore collide more frequently. The presence of a catalyst (allow for accurate collisions) ***Watch out for INERT GASES! They do not speed up reactions. Classic distractor in AP questions. No impact on rate or equilibrium.

AP Chemistry Like Bumper Cars! More COLLISIONS + correct ORIENTATION = Faster Reaction! (and more fun )

AP Chemistry Collision Theory • To overcome coulombic attractions between each bonded atom and the shared electrons, collisions must have enough energy (be forceful enough) with the correct orientation. This is considered a effective collision. • Only a small percentage of the collisions between reactants have enough force and the correct orientation to be effective so reactants need to have lots of collisions in order for reactions to occur. • Increasing the concentration of the reactants, making a larger surface area, or raising the temperature causes more collisions and therefore more effective collisions which increases the rate of reaction.

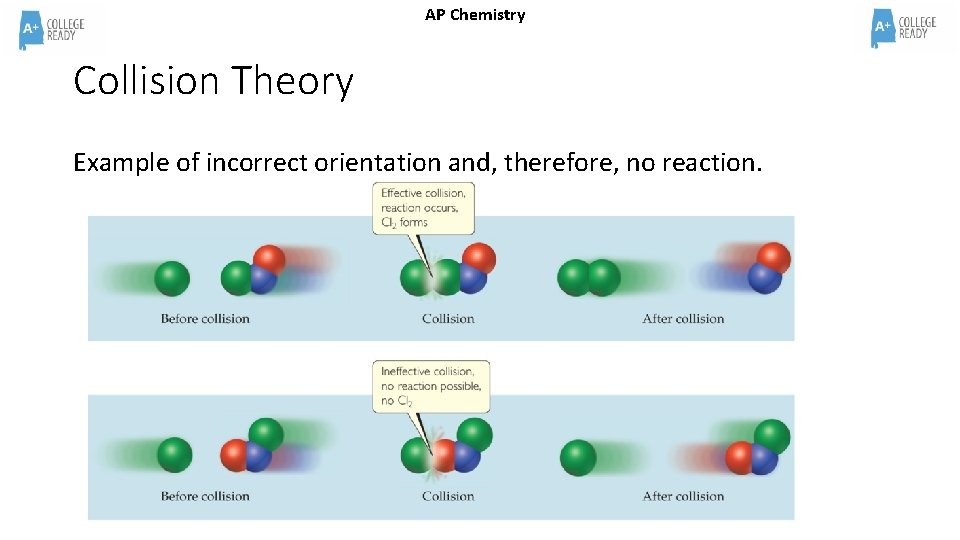
AP Chemistry Collision Theory Example of incorrect orientation and, therefore, no reaction.

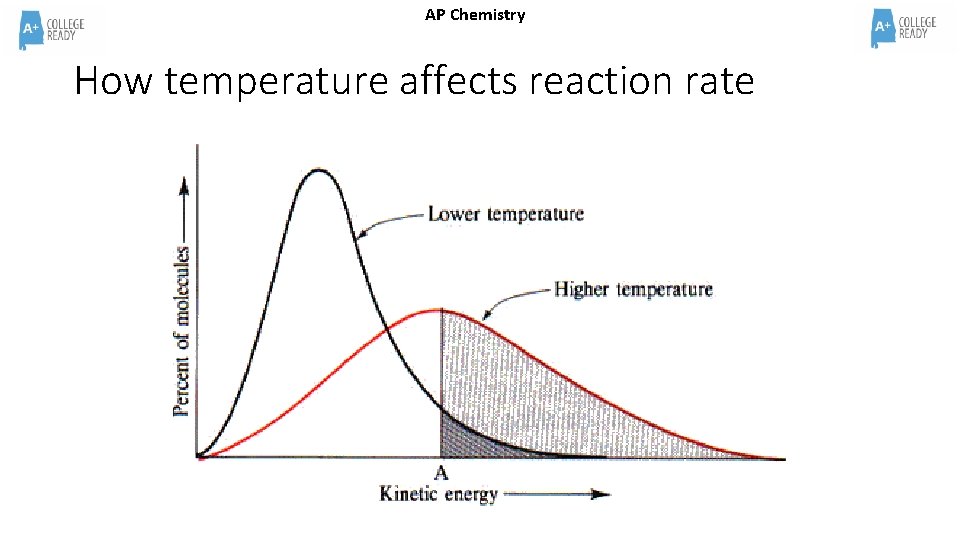
AP Chemistry How temperature affects reaction rate

AP Chemistry Reaction rates The speed of a chemical reactions – the reaction RATE - is the change in concentration over time. Rate = [ ] Time Remember a state function is indicated by a delta ( ) and just means the difference in the final and the initial. Therefore, just subtract the initial concentration from the final concentration and initial time from final time. ** Rates are (+) for products and a (–) sign is used for disappearance of reactants.

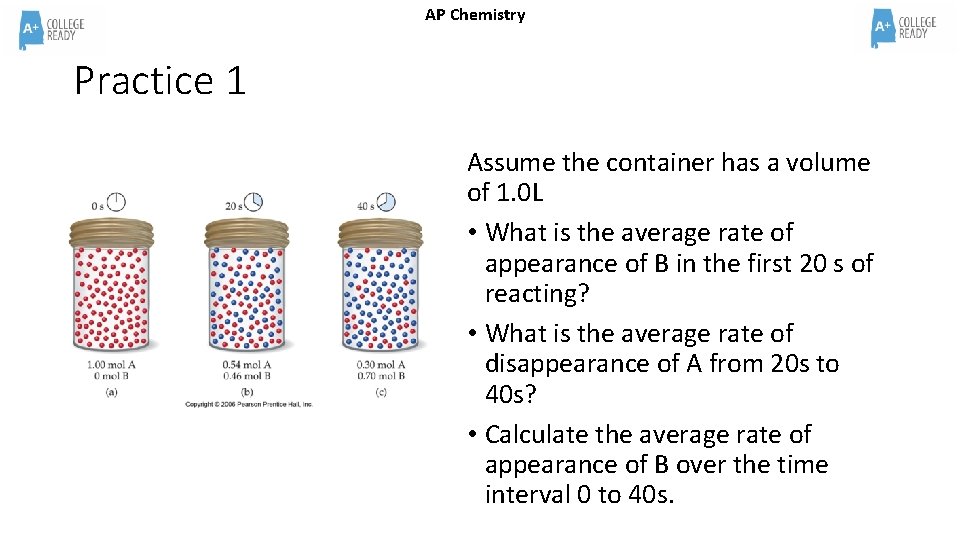
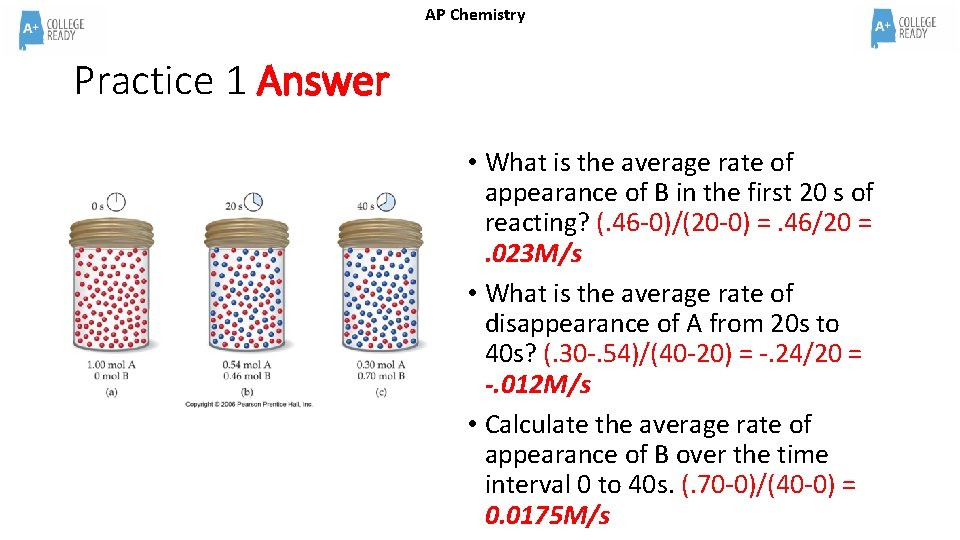
AP Chemistry Practice 1 Assume the container has a volume of 1. 0 L • What is the average rate of appearance of B in the first 20 s of reacting? • What is the average rate of disappearance of A from 20 s to 40 s? • Calculate the average rate of appearance of B over the time interval 0 to 40 s.

AP Chemistry Practice 1 Answer • What is the average rate of appearance of B in the first 20 s of reacting? (. 46 -0)/(20 -0) =. 46/20 =. 023 M/s • What is the average rate of disappearance of A from 20 s to 40 s? (. 30 -. 54)/(40 -20) = -. 24/20 = -. 012 M/s • Calculate the average rate of appearance of B over the time interval 0 to 40 s. (. 70 -0)/(40 -0) = 0. 0175 M/s

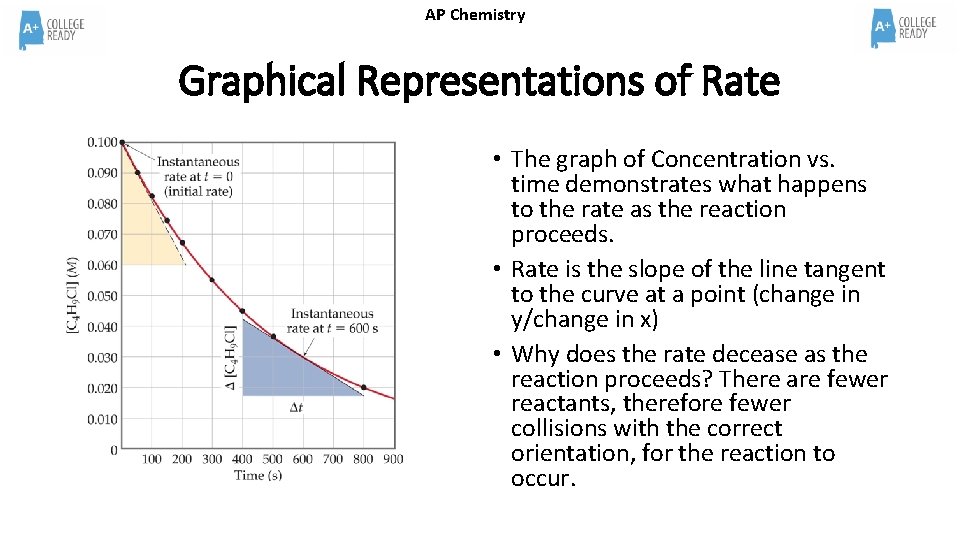
AP Chemistry Graphical Representations of Rate • The graph of Concentration vs. time demonstrates what happens to the rate as the reaction proceeds. • Rate is the slope of the line tangent to the curve at a point (change in y/change in x) • Why does the rate decease as the reaction proceeds? There are fewer reactants, therefore fewer collisions with the correct orientation, for the reaction to occur.

AP Chemistry Reaction Rates and Stoichiometry Instantaneous Rates of Reaction

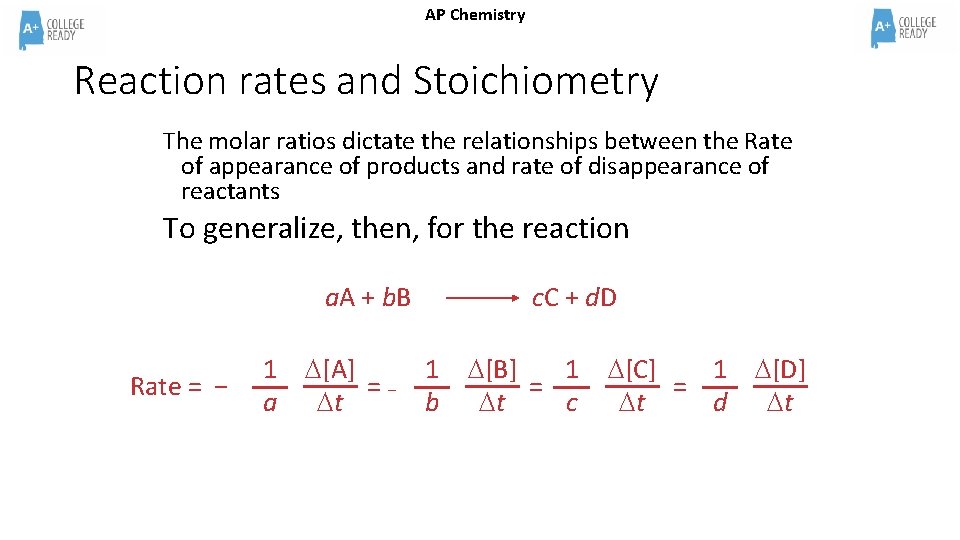
AP Chemistry Reaction rates and Stoichiometry The molar ratios dictate the relationships between the Rate of appearance of products and rate of disappearance of reactants To generalize, then, for the reaction a. A + b. B Rate = − 1 [A] =− a t c. C + d. D 1 b [B] 1 = t c [C] 1 [D] = t d t

AP Chemistry Example 2 • For the reaction A B the Rate of the disappearance of A = the rate of appearance of B, because they are in a 1: 1 molar ratio • For the reaction C 2 D the rate of the disappearance of C = ½ the rate of the appearance of D, because they are in a 1 C: 2 D ratio or you could say D’s rate is 2 X the rate of C’s (basically, D is the Faster one because the coefficient is bigger!)

AP Chemistry Practice 2 • For the reaction Q 4 X • Which component has a faster rate? • How much faster? • So in comparison, what would you say about the slower component rate?

AP Chemistry Practice 2 Answer • For the reaction Q 4 X • Which component has a faster rate? X • How much faster? 4 times faster • So, in comparison, what would you say about the slower component rate? ¼ the rate of X

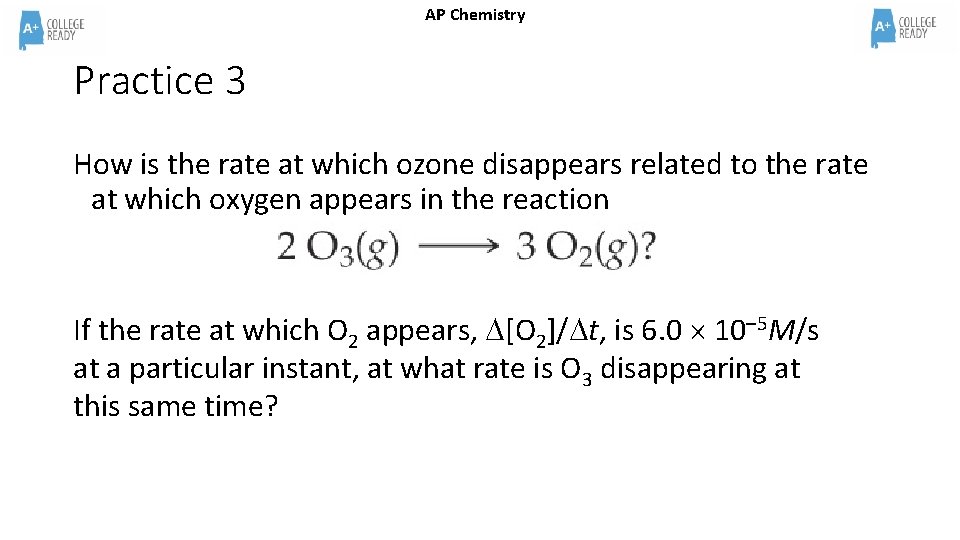
AP Chemistry Practice 3 How is the rate at which ozone disappears related to the rate at which oxygen appears in the reaction If the rate at which O 2 appears, [O 2]/ t, is 6. 0 10– 5 M/s at a particular instant, at what rate is O 3 disappearing at this same time?

AP Chemistry Practice 3 Answer How is the rate at which ozone disappears related to the rate at which oxygen appears in the reaction If the rate at which O 2 appears, [O 2]/ t, is 6. 0 10– 5 M/s at a particular instant, at what rate is O 3 disappearing at this same time? The rate of O 3 is 2/3 the rate of O 2 therefore the rate of the O 3 at this instant is 4 x 10 -5 M/s 6. 0 10– 5 M/s x 3 mol O 3 = 4 x 10 -5 M/s 2 mol O 2

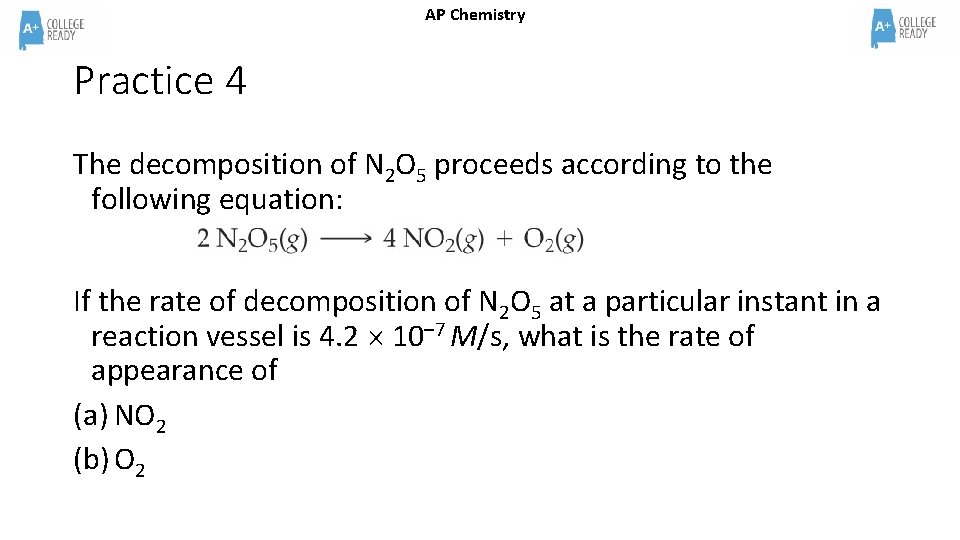
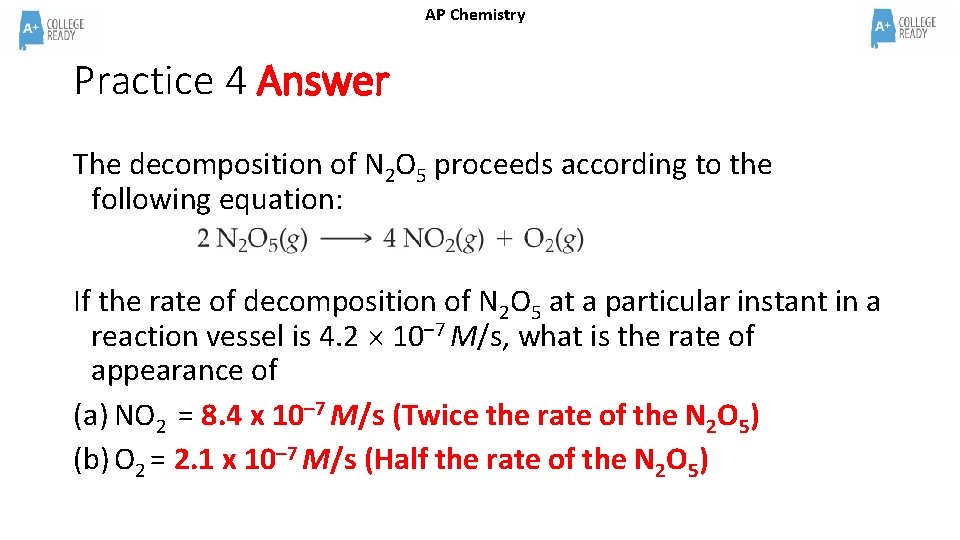
AP Chemistry Practice 4 The decomposition of N 2 O 5 proceeds according to the following equation: If the rate of decomposition of N 2 O 5 at a particular instant in a reaction vessel is 4. 2 10– 7 M/s, what is the rate of appearance of (a) NO 2 (b) O 2

AP Chemistry Practice 4 Answer The decomposition of N 2 O 5 proceeds according to the following equation: If the rate of decomposition of N 2 O 5 at a particular instant in a reaction vessel is 4. 2 10– 7 M/s, what is the rate of appearance of (a) NO 2 = 8. 4 x 10– 7 M/s (Twice the rate of the N 2 O 5) (b) O 2 = 2. 1 x 10– 7 M/s (Half the rate of the N 2 O 5)

AP Chemistry Concentration and Rate Laws The Differential Rate Law

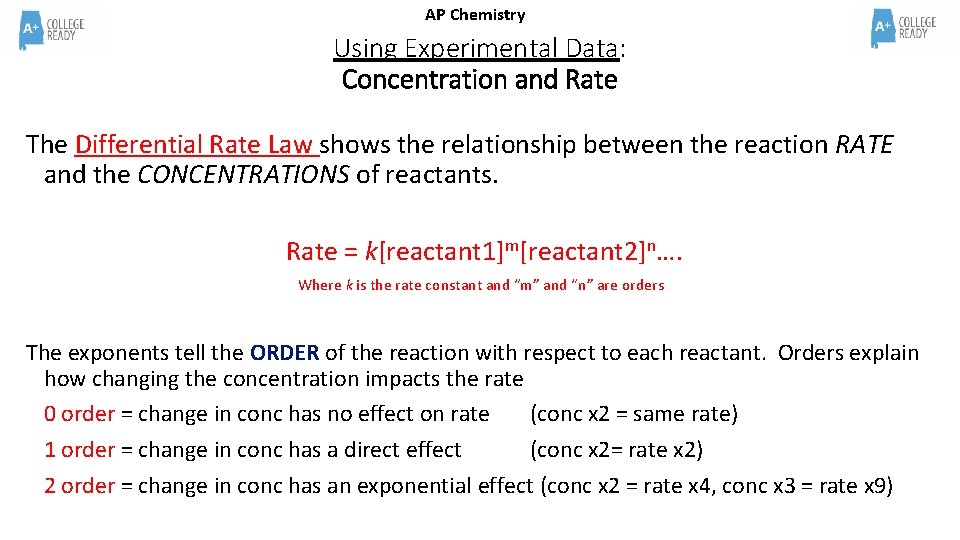
AP Chemistry Using Experimental Data: Concentration and Rate The Differential Rate Law shows the relationship between the reaction RATE and the CONCENTRATIONS of reactants. Rate = k[reactant 1]m[reactant 2]n…. Where k is the rate constant and “m” and “n” are orders The exponents tell the ORDER of the reaction with respect to each reactant. Orders explain how changing the concentration impacts the rate 0 order = change in conc has no effect on rate (conc x 2 = same rate) 1 order = change in conc has a direct effect (conc x 2= rate x 2) 2 order = change in conc has an exponential effect (conc x 2 = rate x 4, conc x 3 = rate x 9)

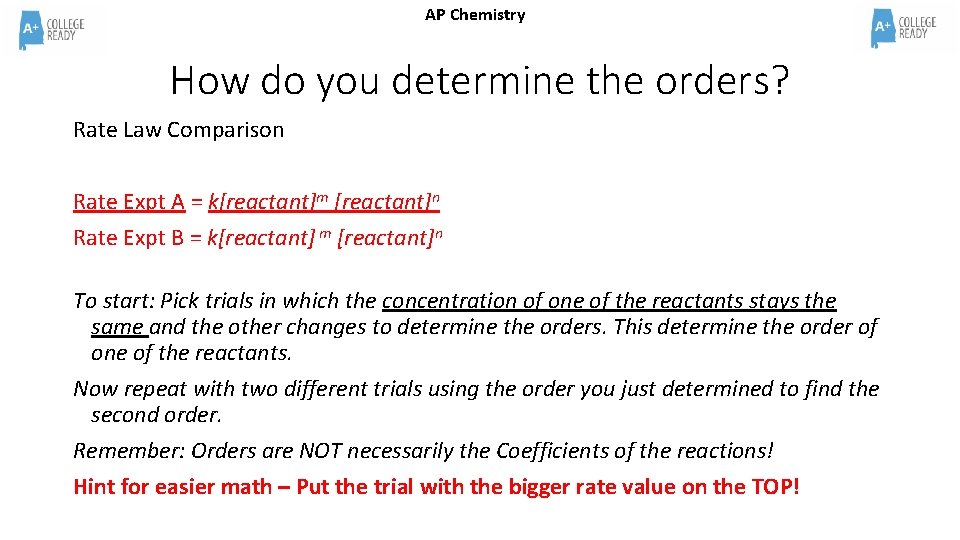
AP Chemistry How do you determine the orders? Rate Law Comparison Rate Expt A = k[reactant]m [reactant]n Rate Expt B = k[reactant] m [reactant]n To start: Pick trials in which the concentration of one of the reactants stays the same and the other changes to determine the orders. This determine the order of one of the reactants. Now repeat with two different trials using the order you just determined to find the second order. Remember: Orders are NOT necessarily the Coefficients of the reactions! Hint for easier math – Put the trial with the bigger rate value on the TOP!

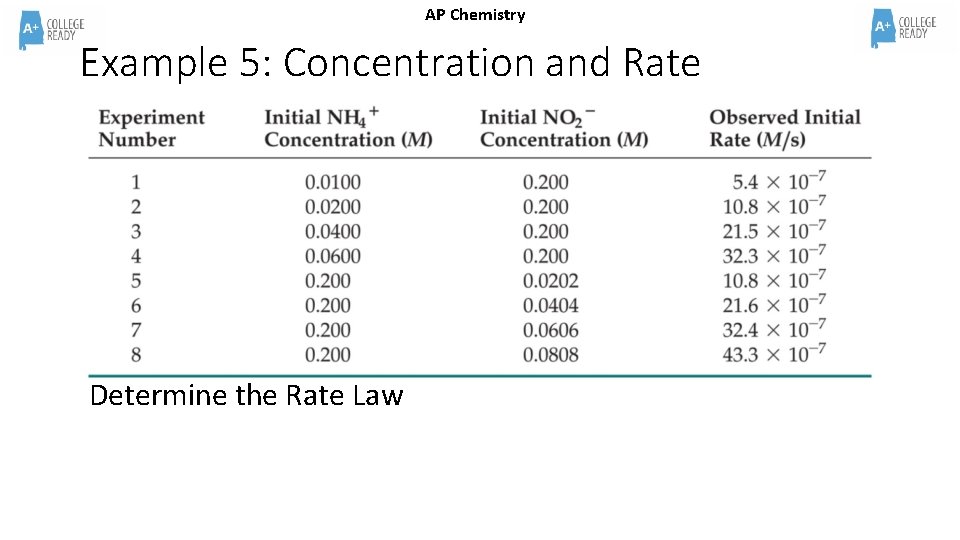
AP Chemistry Example 5: Concentration and Rate Determine the Rate Law

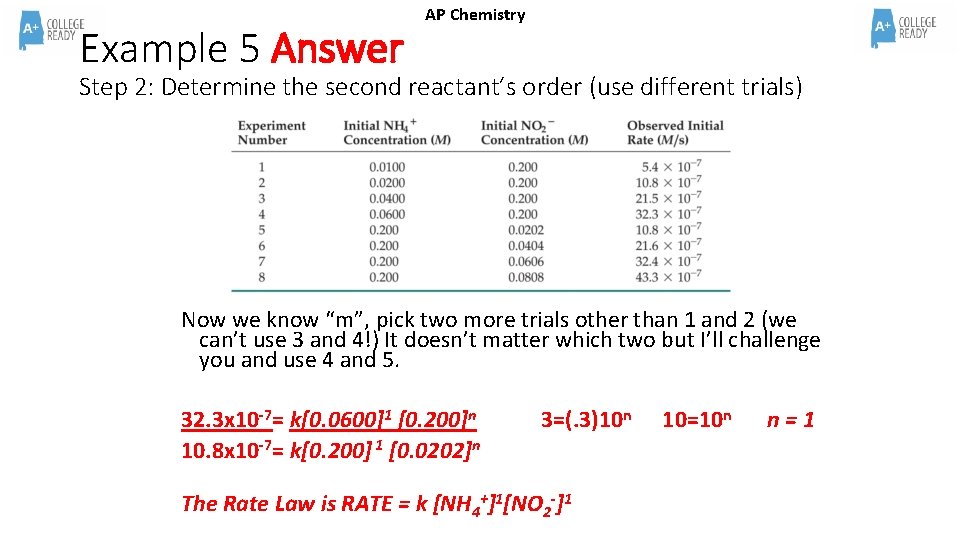
Example 5 Answer AP Chemistry Step 1: Determine the first reactant’s order (Pick one that stays the same) NH 4+(aq) + NO 2−(aq) N 2(g) + 2 H 2 O(l) Comparing Experiments 1 and 2, when [NO 2 -] stays the same. 10. 8 x 10 -7= k[0. 0200]m [0. 200]n 2=2 m m=1 5. 4 x 10 -7 = k[0. 0100] m [0. 200]n

Example 5 Answer AP Chemistry Step 2: Determine the second reactant’s order (use different trials) Now we know “m”, pick two more trials other than 1 and 2 (we can’t use 3 and 4!) It doesn’t matter which two but I’ll challenge you and use 4 and 5. 32. 3 x 10 -7= k[0. 0600]1 [0. 200]n 10. 8 x 10 -7= k[0. 200] 1 [0. 0202]n 3=(. 3)10 n The Rate Law is RATE = k [NH 4+]1[NO 2 -]1 10=10 n n=1
![AP Chemistry Concentration and Rate This means Rate NH 4 Rate NO AP Chemistry Concentration and Rate • This means Rate [NH 4+] − Rate [NO](https://slidetodoc.com/presentation_image_h2/6629d4963cb673a62499d3c5c0aa1eae/image-28.jpg)
AP Chemistry Concentration and Rate • This means Rate [NH 4+] − Rate [NO 2 ] Rate [NH+] [NO 2−] or Rate = k [NH 4+] [NO 2−] • This equation is called the rate law, and k is the rate constant.

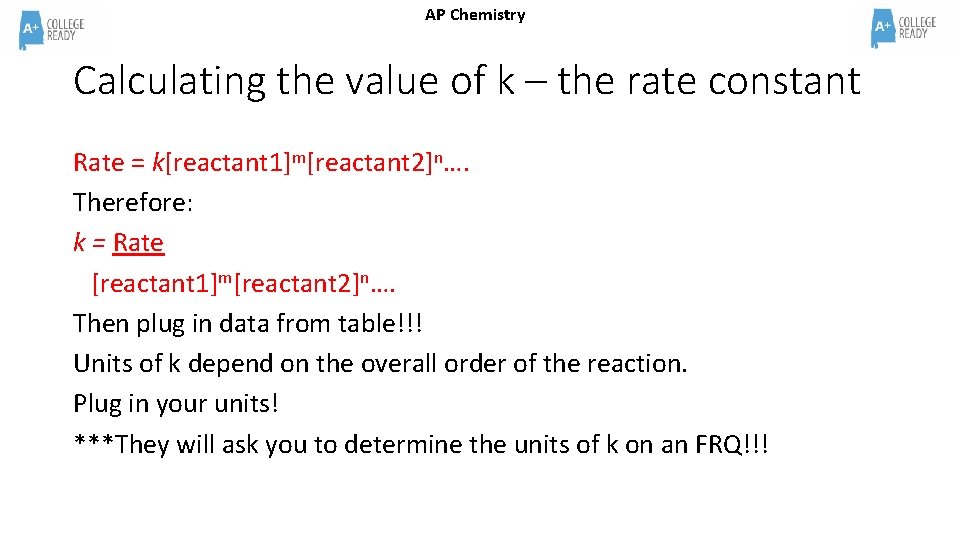
AP Chemistry Calculating the value of k – the rate constant Rate = k[reactant 1]m[reactant 2]n…. Therefore: k = Rate [reactant 1]m[reactant 2]n…. Then plug in data from table!!! Units of k depend on the overall order of the reaction. Plug in your units! ***They will ask you to determine the units of k on an FRQ!!!

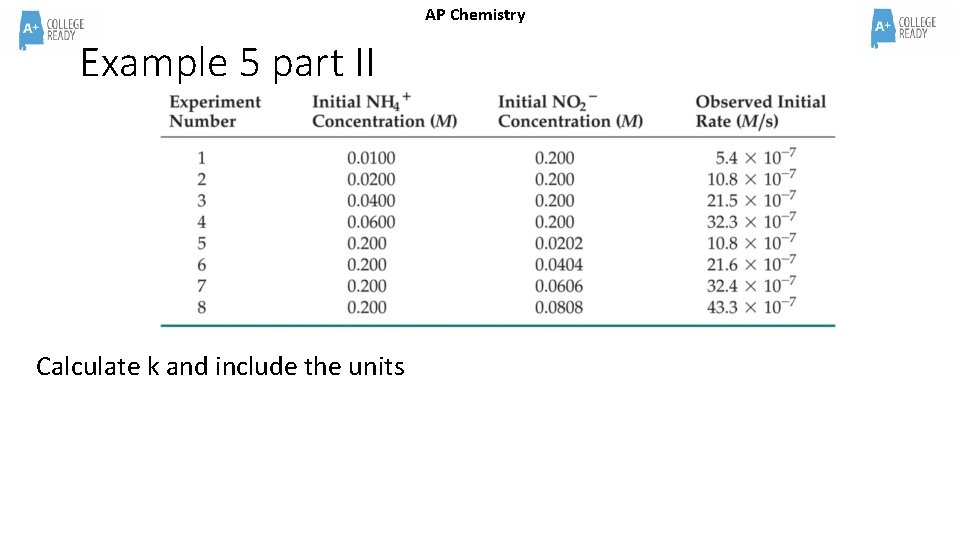
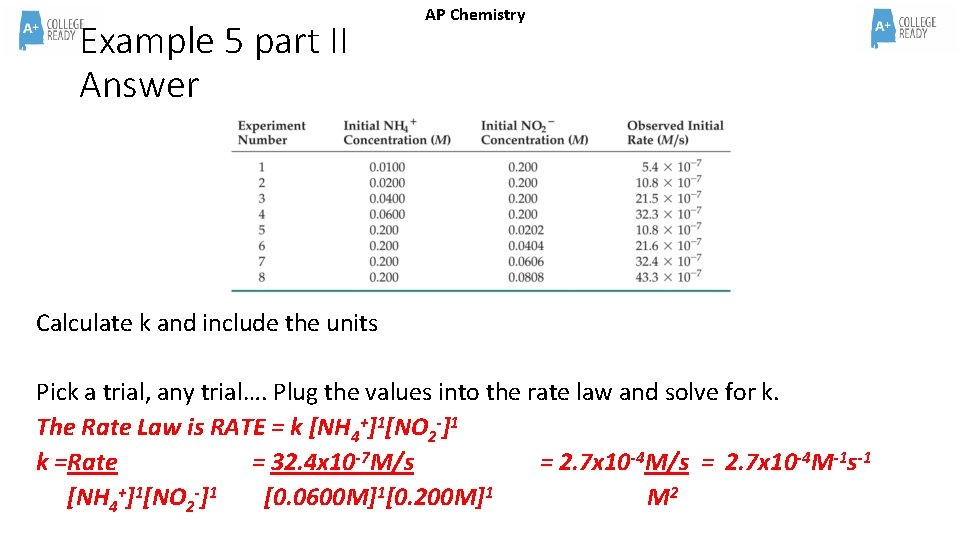
AP Chemistry Example 5 part II Calculate k and include the units

Example 5 part II Answer AP Chemistry Calculate k and include the units Pick a trial, any trial…. Plug the values into the rate law and solve for k. The Rate Law is RATE = k [NH 4+]1[NO 2 -]1 k =Rate = 32. 4 x 10 -7 M/s = 2. 7 x 10 -4 M-1 s-1 [NH 4+]1[NO 2 -]1 [0. 0600 M]1[0. 200 M]1 M 2

AP Chemistry Overall order of the reaction • Overall order = sum of the orders of the reactants Rate = k[reactant 1]m[reactant 2]n…. Overall order = m+n+……

AP Chemistry Differential Rate Law Questions – What’s next? • Do you know they relationships between concentration and rate and how they determine the orders? • Can you determine a rate law using given data? • Can you calculate a rate constant? • Can you determine the overall order? Now: • Can you determine a rate or a concentration if you have established the rate law and calculated the k? (plug it in, plug it in!) • Can you determine a rate law without a calculator? (think multiple choice and the relationships between rate and concentration)

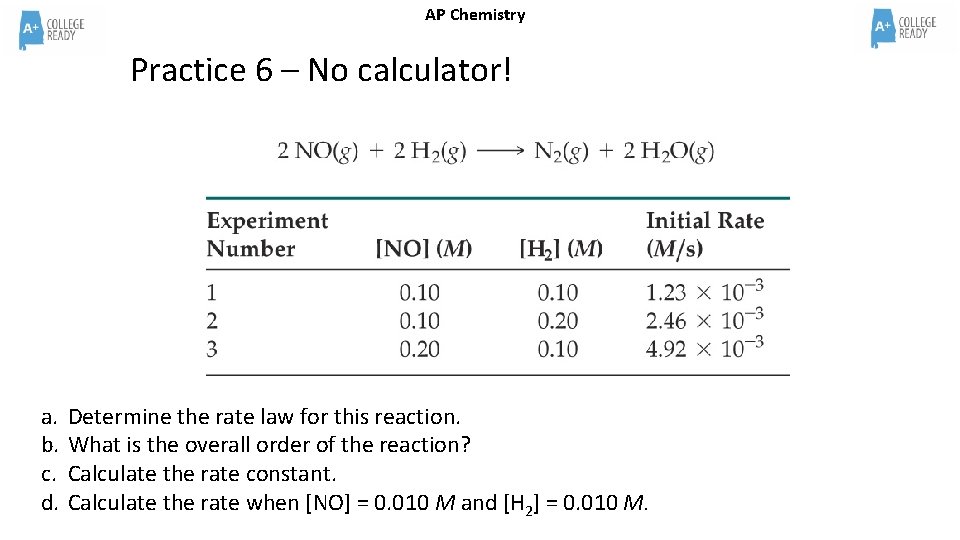
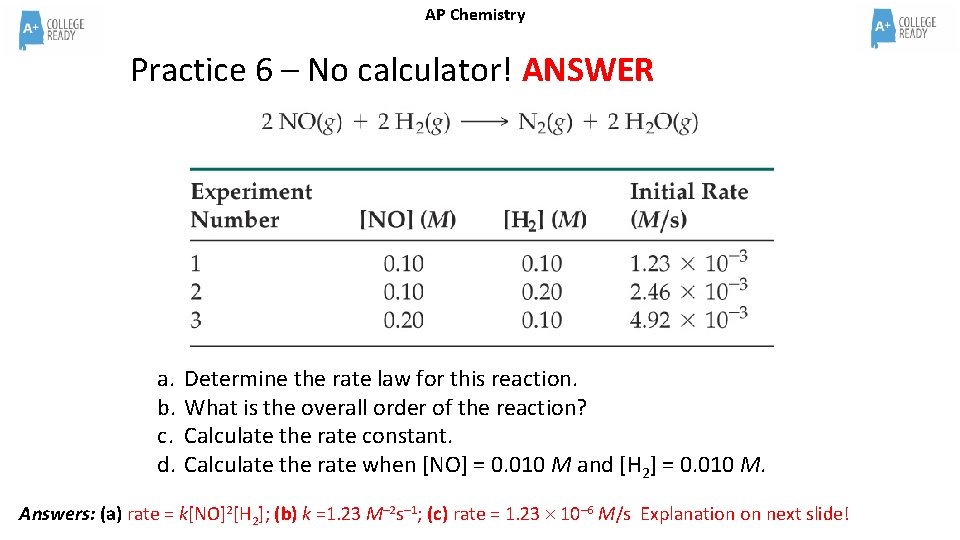
AP Chemistry Practice 6 – No calculator! a. b. c. d. Determine the rate law for this reaction. What is the overall order of the reaction? Calculate the rate constant. Calculate the rate when [NO] = 0. 010 M and [H 2] = 0. 010 M.

AP Chemistry Practice 6 – No calculator! ANSWER a. b. c. d. Determine the rate law for this reaction. What is the overall order of the reaction? Calculate the rate constant. Calculate the rate when [NO] = 0. 010 M and [H 2] = 0. 010 M. Answers: (a) rate = k[NO]2[H 2]; (b) k =1. 23 M– 2 s– 1; (c) rate = 1. 23 10– 6 M/s Explanation on next slide!

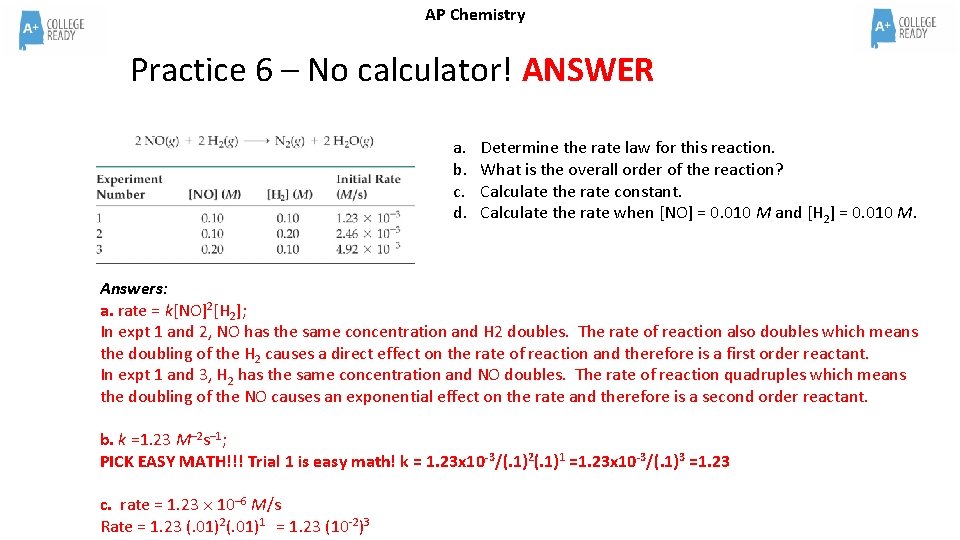
AP Chemistry Practice 6 – No calculator! ANSWER a. b. c. d. Determine the rate law for this reaction. What is the overall order of the reaction? Calculate the rate constant. Calculate the rate when [NO] = 0. 010 M and [H 2] = 0. 010 M. Answers: a. rate = k[NO]2[H 2]; In expt 1 and 2, NO has the same concentration and H 2 doubles. The rate of reaction also doubles which means the doubling of the H 2 causes a direct effect on the rate of reaction and therefore is a first order reactant. In expt 1 and 3, H 2 has the same concentration and NO doubles. The rate of reaction quadruples which means the doubling of the NO causes an exponential effect on the rate and therefore is a second order reactant. b. k =1. 23 M– 2 s– 1; PICK EASY MATH!!! Trial 1 is easy math! k = 1. 23 x 10 -3/(. 1)2(. 1)1 =1. 23 x 10 -3/(. 1)3 =1. 23 c. rate = 1. 23 10– 6 M/s Rate = 1. 23 (. 01)2(. 01)1 = 1. 23 (10 -2)3

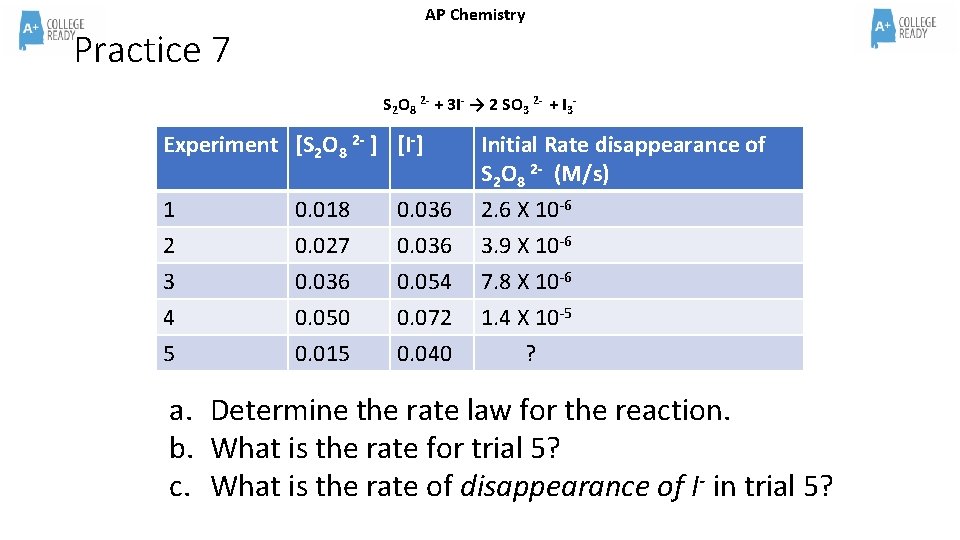
AP Chemistry Practice 7 S 2 O 8 2 - + 3 I- → 2 SO 3 2 - + I 3 - Experiment [S 2 O 8 2 - ] [I-] 1 0. 018 0. 036 Initial Rate disappearance of S 2 O 8 2 - (M/s) 2. 6 X 10 -6 2 3 4 5 0. 027 0. 036 0. 050 0. 015 0. 036 0. 054 0. 072 0. 040 3. 9 X 10 -6 7. 8 X 10 -6 1. 4 X 10 -5 ? a. Determine the rate law for the reaction. b. What is the rate for trial 5? c. What is the rate of disappearance of I- in trial 5?

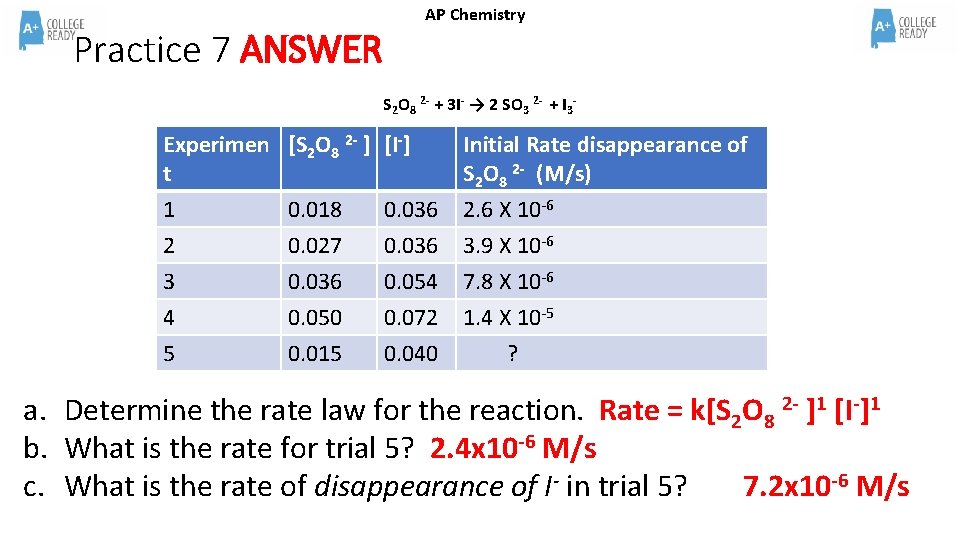
Practice 7 ANSWER AP Chemistry S 2 O 8 2 - + 3 I- → 2 SO 3 2 - + I 3 - Experimen [S 2 O 8 2 - ] [I-] t 1 0. 018 0. 036 Initial Rate disappearance of S 2 O 8 2 - (M/s) 2. 6 X 10 -6 2 3 4 5 3. 9 X 10 -6 7. 8 X 10 -6 1. 4 X 10 -5 ? 0. 027 0. 036 0. 050 0. 015 0. 036 0. 054 0. 072 0. 040 a. Determine the rate law for the reaction. Rate = k[S 2 O 8 2 - ]1 [I-]1 b. What is the rate for trial 5? 2. 4 x 10 -6 M/s c. What is the rate of disappearance of I- in trial 5? 7. 2 x 10 -6 M/s

AP Chemistry The Change of Concentration with Time The Integrated Rate Law

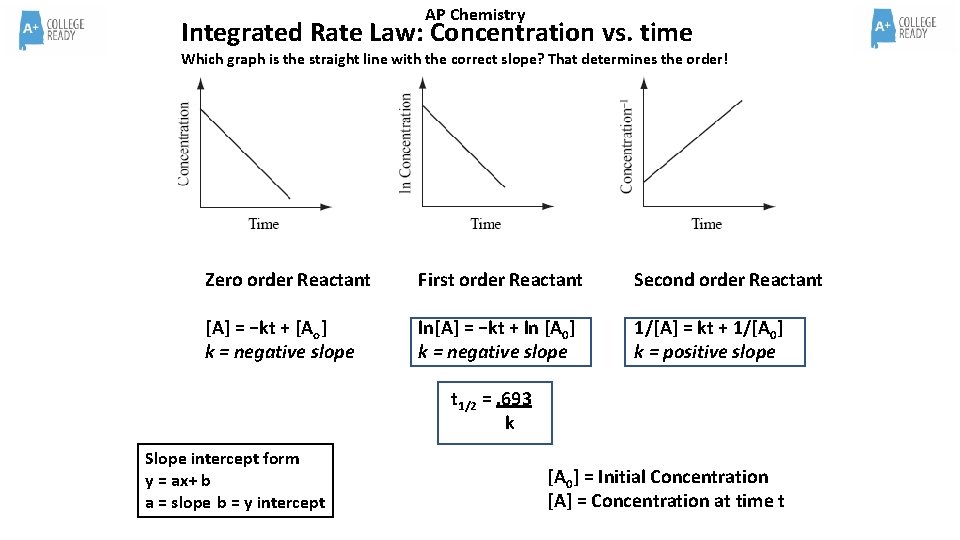
AP Chemistry Integrated Rate Law When concentration and time data is analyzed graphically, a linear relationship arises based on the order of the reactant. Zero order reactants show a linear relationship in a graph of concentration vs. time ([ ] vs. t) First order reactant show a linear relationship in a graph of the natural log of concentration vs. time (ln [ ] vs. t) Second order reactants show a linear relationship in a graph of the inverse of concentration vs. time (1/[ ] vs. t) For these types of questions, two or three graphs will be presented and the graph with the straight line (a linear relationship!) will be the graph that determines the order. Your job is to recognize the straight line and know the graph to order relationship!

AP Chemistry Integrated Rate Law: Concentration vs. time Which graph is the straight line with the correct slope? That determines the order! Zero order Reactant First order Reactant Second order Reactant [A] = −kt + [Ao] k = negative slope ln[A] = −kt + ln [A 0] k = negative slope 1/[A] = kt + 1/[A 0] k = positive slope t 1/2 =. 693 k Slope intercept form y = ax+ b a = slope b = y intercept [A 0] = Initial Concentration [A] = Concentration at time t

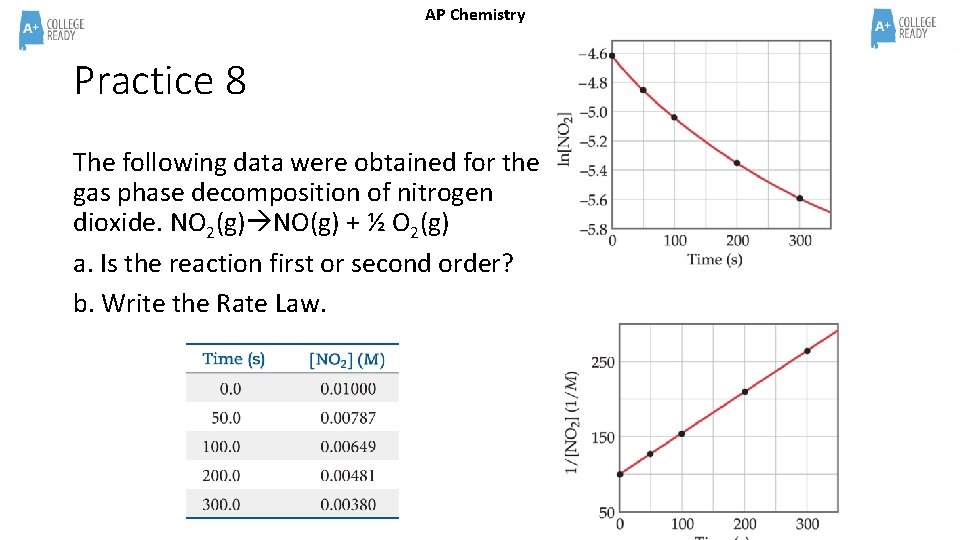
AP Chemistry Practice 8 The following data were obtained for the gas phase decomposition of nitrogen dioxide. NO 2(g) NO(g) + ½ O 2(g) a. Is the reaction first or second order? b. Write the Rate Law.

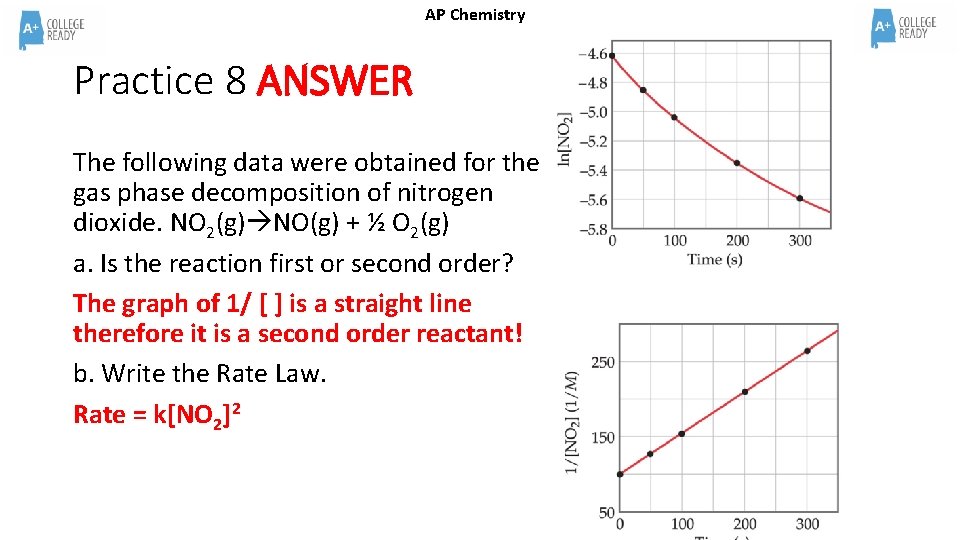
AP Chemistry Practice 8 ANSWER The following data were obtained for the gas phase decomposition of nitrogen dioxide. NO 2(g) NO(g) + ½ O 2(g) a. Is the reaction first or second order? The graph of 1/ [ ] is a straight line therefore it is a second order reactant! b. Write the Rate Law. Rate = k[NO 2]2

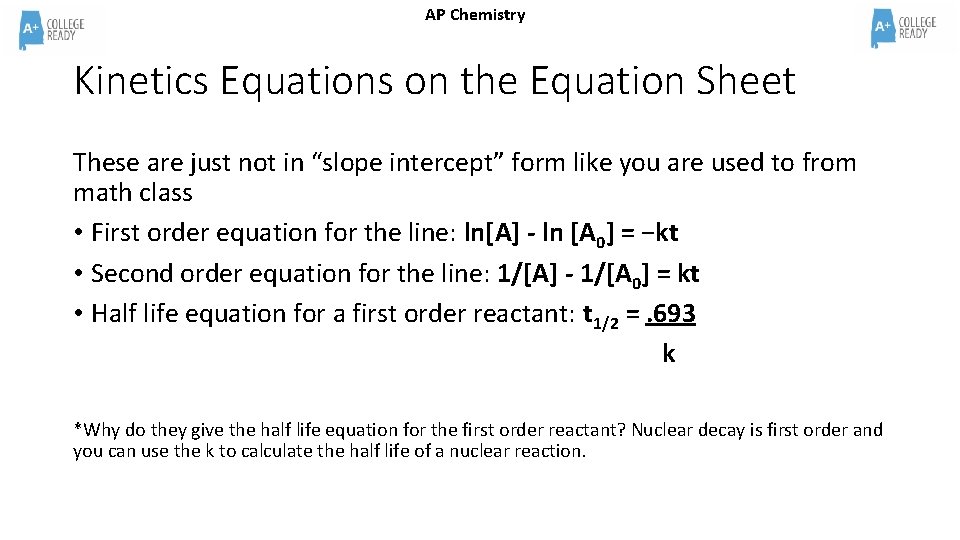
AP Chemistry Kinetics Equations on the Equation Sheet These are just not in “slope intercept” form like you are used to from math class • First order equation for the line: ln[A] - ln [A 0] = −kt • Second order equation for the line: 1/[A] - 1/[A 0] = kt • Half life equation for a first order reactant: t 1/2 =. 693 k *Why do they give the half life equation for the first order reactant? Nuclear decay is first order and you can use the k to calculate the half life of a nuclear reaction.

AP Chemistry Practice 9 For the decomposition of NO 2 discussed in Practice 8: a. What is the value of k? b. If the initial concentration of NO 2 in a closed vessel is 0. 500 M, what is the concentration of this reactant after 0. 500 hours?

AP Chemistry Practice 9 ANSWER For the decomposition of NO 2 discussed in Practice 8: What is the value of k? Use the equations of the equation sheet!!! 1/0. 00380 -1/0. 01000 = k(300 s) (From the Data Table!) k=. 543 M-1 s-1 If the initial concentration of NO 2 in a closed vessel is 0. 500 M, what is the concentration of this reactant after 0. 500 hours? 1/[A] - 1/[A 0] = kt 1/[A] = (. 543 M-1 s-1)(1800 s) + 1/[0. 500] = 979. 4 [A] = 1. 02 x 10 -3 M

AP Chemistry Reactions, on the molecular level occur in several steps Mechanisms

AP Chemistry Determining the rate law from a mechanism Series of elementary steps must satisfy two requirements: 1. The sum of the elementary steps must equal the overall balanced equation for the reaction 2. The mechanism must agree with the experimentally determined rate law The rate law is determined by the sum of the rate laws for all steps THROUGH the SLOW step.

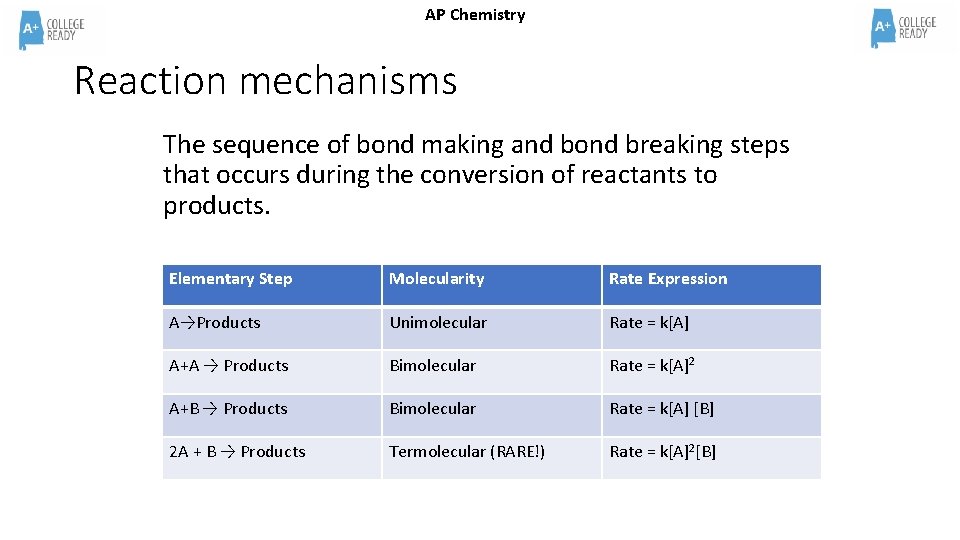
AP Chemistry Reaction mechanisms The sequence of bond making and bond breaking steps that occurs during the conversion of reactants to products. Elementary Step Molecularity Rate Expression A→Products Unimolecular Rate = k[A] A+A → Products Bimolecular Rate = k[A]2 A+B → Products Bimolecular Rate = k[A] [B] 2 A + B → Products Termolecular (RARE!) Rate = k[A]2[B]

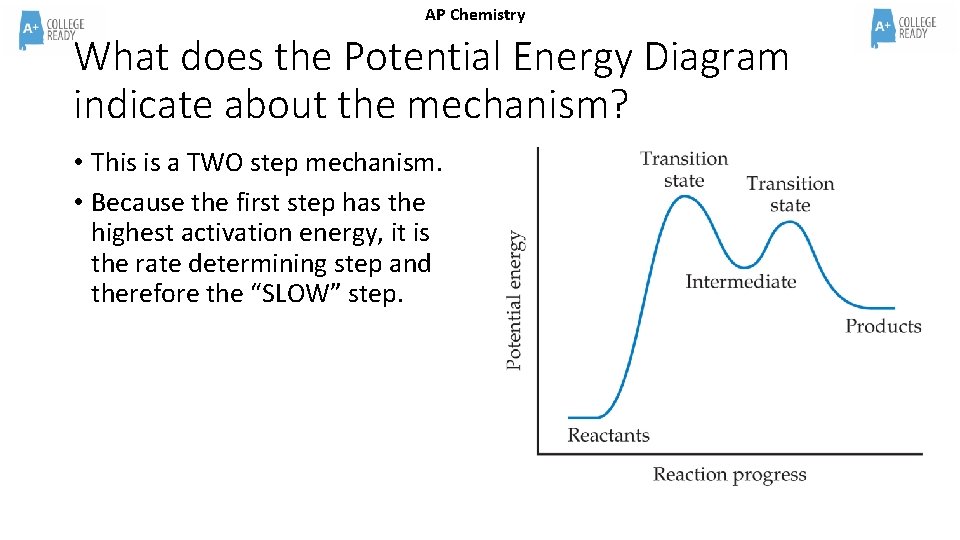
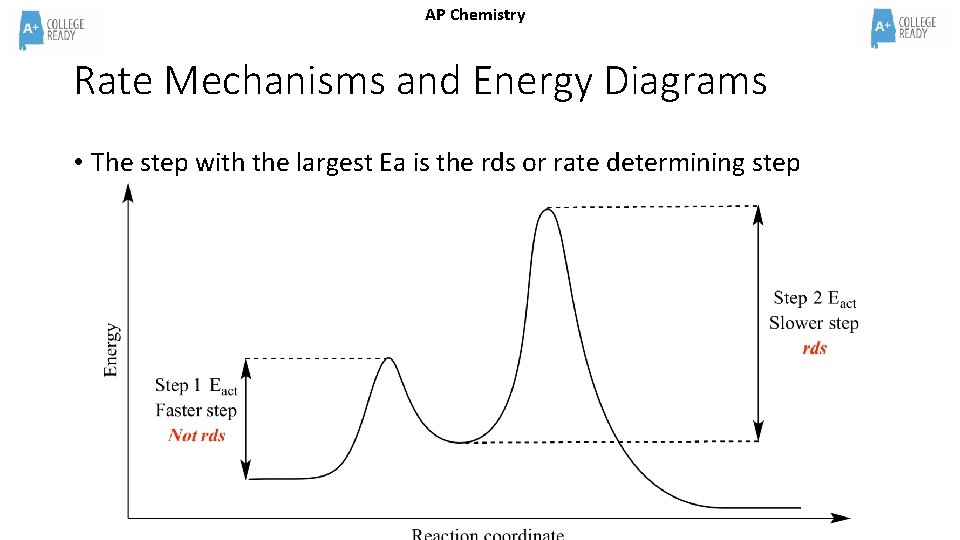
AP Chemistry What does the Potential Energy Diagram indicate about the mechanism? • This is a TWO step mechanism. • Because the first step has the highest activation energy, it is the rate determining step and therefore the “SLOW” step.

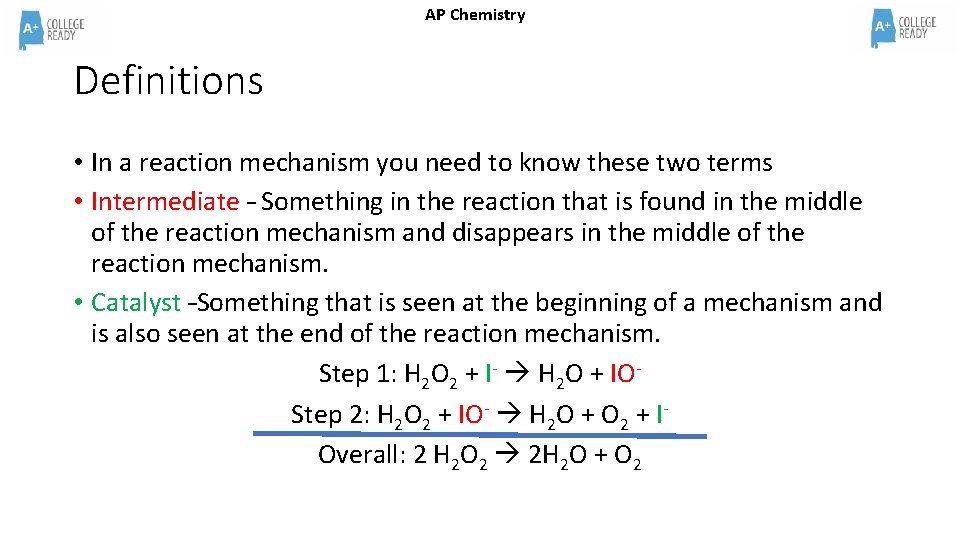
AP Chemistry Definitions • In a reaction mechanism you need to know these two terms • Intermediate – Something in the reaction that is found in the middle of the reaction mechanism and disappears in the middle of the reaction mechanism. • Catalyst –Something that is seen at the beginning of a mechanism and is also seen at the end of the reaction mechanism. Step 1: H 2 O 2 + I- H 2 O + IOStep 2: H 2 O 2 + IO- H 2 O + O 2 + IOverall: 2 H 2 O 2 2 H 2 O + O 2

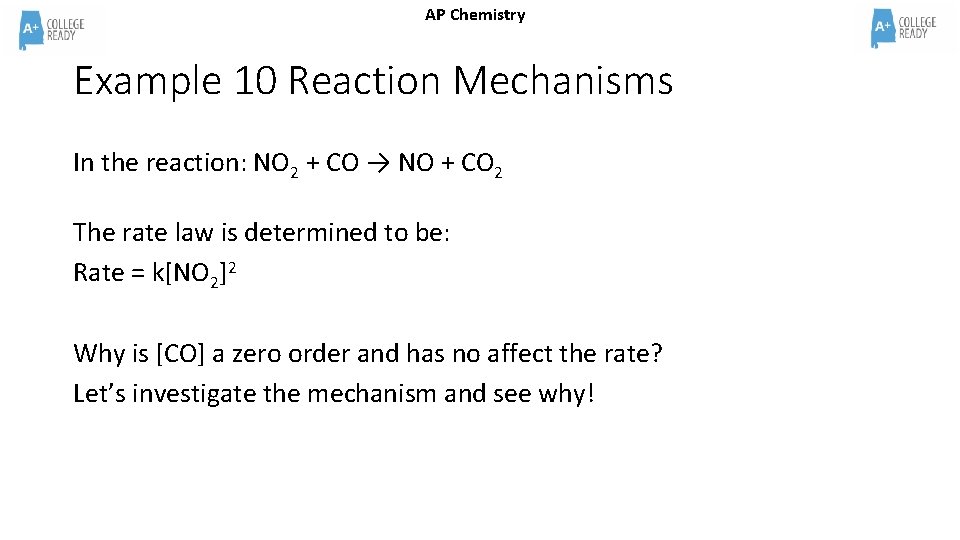
AP Chemistry Example 10 Reaction Mechanisms In the reaction: NO 2 + CO → NO + CO 2 The rate law is determined to be: Rate = k[NO 2]2 Why is [CO] a zero order and has no affect the rate? Let’s investigate the mechanism and see why!

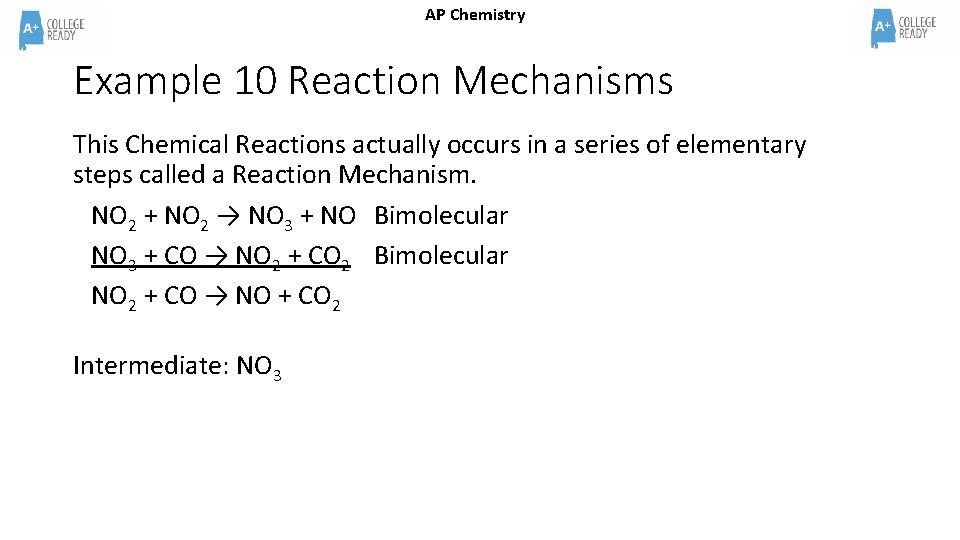
AP Chemistry Example 10 Reaction Mechanisms This Chemical Reactions actually occurs in a series of elementary steps called a Reaction Mechanism. NO 2 + NO 2 → NO 3 + NO Bimolecular NO 3 + CO → NO 2 + CO 2 Bimolecular NO 2 + CO → NO + CO 2 Intermediate: NO 3

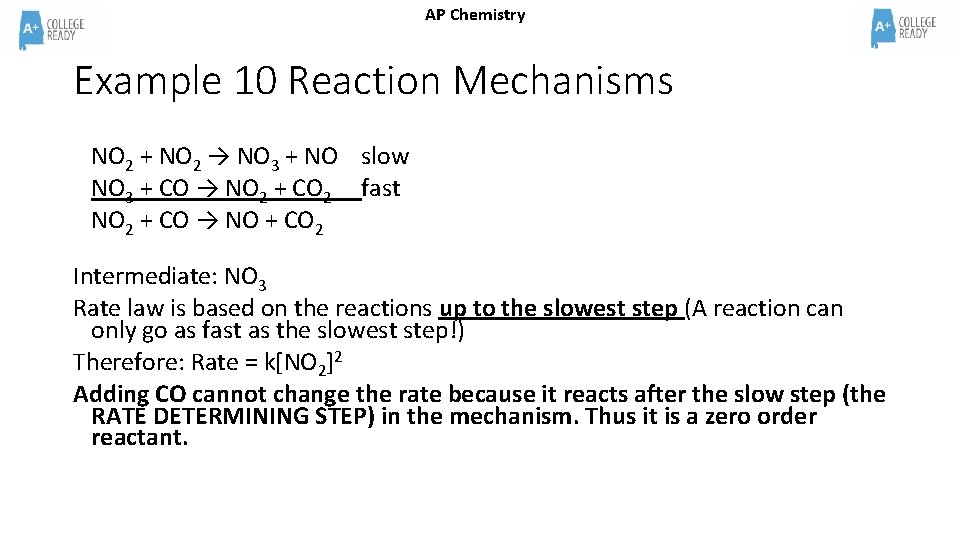
AP Chemistry Example 10 Reaction Mechanisms NO 2 + NO 2 → NO 3 + NO slow NO 3 + CO → NO 2 + CO 2 fast NO 2 + CO → NO + CO 2 Intermediate: NO 3 Rate law is based on the reactions up to the slowest step (A reaction can only go as fast as the slowest step!) Therefore: Rate = k[NO 2]2 Adding CO cannot change the rate because it reacts after the slow step (the RATE DETERMINING STEP) in the mechanism. Thus it is a zero order reactant.

AP Chemistry Kinds of AP questions on Mechanisms • Pick the correct mechanism: • Given a rate law (or if you determined one in an FRQ), pick from several proposed mechanisms to identify the one that matches the rate law requirements (matches the orders based on the slow step) • Is the mechanism correct? • To be a correct mechanism the steps must add up to the overall reaction AND • Does the mechanism match the rate law? Hint – DRAW a line after the slow step in the mechanism and add up the number of the reactants in each of the step before and including the slow step. The number should match their order. Watch out for intermediates and catalysts!! They should cancel out.

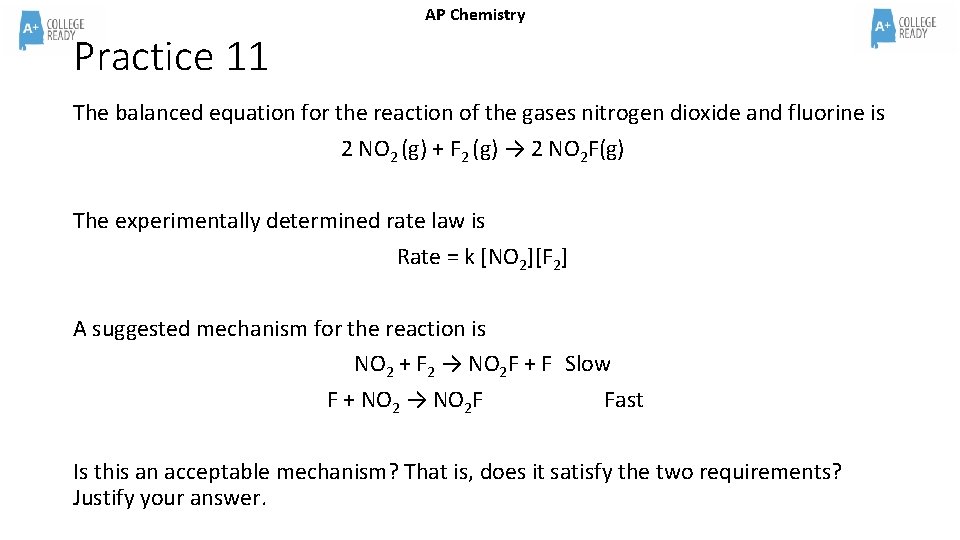
AP Chemistry Practice 11 The balanced equation for the reaction of the gases nitrogen dioxide and fluorine is 2 NO 2 (g) + F 2 (g) → 2 NO 2 F(g) The experimentally determined rate law is Rate = k [NO 2][F 2] A suggested mechanism for the reaction is NO 2 + F 2 → NO 2 F + F Slow F + NO 2 → NO 2 F Fast Is this an acceptable mechanism? That is, does it satisfy the two requirements? Justify your answer.

AP Chemistry Practice 11 ANSWER The balanced equation for the reaction of the gases nitrogen dioxide and fluorine is 2 NO 2 (g) + F 2 (g) → 2 NO 2 F(g) The experimentally determined rate law is Rate = k [NO 2][F 2] A suggested mechanism for the reaction is NO 2 + F 2 → NO 2 F + F Slow F + NO 2 → NO 2 F Fast Is this an acceptable mechanism? That is, does it satisfy the two requirements? Justify your answer. Yes, this an acceptable mechanism. The reaction matches the overall chemical equation and, because the slow step is the first step, the mechanism matches the rate law. F is an intermediate. If the second step in the mechanism were the slow step, then the rate law would have been: Rate = k [NO 2]2 [F 2] making NO 2 a second order reactant which does not match given rate law.

AP Chemistry Catalysts Making collisions effective

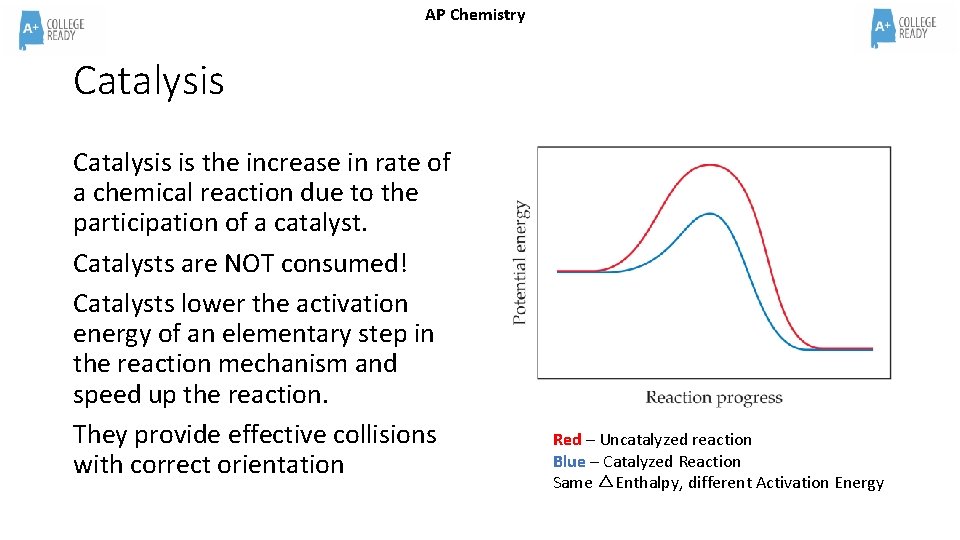
AP Chemistry Catalysis is the increase in rate of a chemical reaction due to the participation of a catalyst. Catalysts are NOT consumed! Catalysts lower the activation energy of an elementary step in the reaction mechanism and speed up the reaction. They provide effective collisions with correct orientation Red – Uncatalyzed reaction Blue – Catalyzed Reaction Same △Enthalpy, different Activation Energy

AP Chemistry Types of Catalysts 1. 2. 3. 4. Homogenous/Heterogenous Catalysts (STATES of Matter) Acid/Base Catalysts Surface Catalysts (Hydrocarbon Cracking) Biological Catalysts (Enzymes)

AP Chemistry Catalysts in Nature How Chlorofluorocarbons break down in the presence of light: CCl 2 F 2 (g) → CCl. F 2 (g) + Cl(g) What Happens to the Ozone layer: Cl(g) + O 3(g) → Cl. O(g) + O 2 (g) O 3(g) + Cl. O(g) → Cl(g) + 2 O 2 (g) Cl is a catalyst in the destruction of the ozone layer. It is used but comes back out unchanged. Cl. O(g) is an intermediate. It shows up in the middle of the reaction and disappears before the end.

AP Chemistry Rate Mechanisms and Energy Diagrams • The step with the largest Ea is the rds or rate determining step