AP Chemistry Basics of Chemical Bonding Properties of
AP Chemistry Basics of Chemical Bonding Properties of substances are largely dependent on the bonds holding the material together.
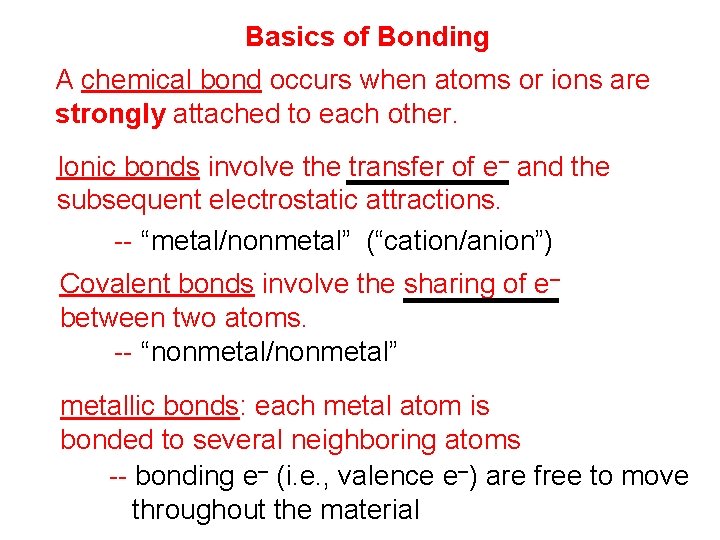
Basics of Bonding A chemical bond occurs when atoms or ions are strongly attached to each other. Ionic bonds involve the transfer of e– and the subsequent electrostatic attractions. -- “metal/nonmetal” (“cation/anion”) Covalent bonds involve the sharing of e– between two atoms. -- “nonmetal/nonmetal” metallic bonds: each metal atom is bonded to several neighboring atoms -- bonding e– (i. e. , valence e–) are free to move throughout the material

Lewis symbols show ONLY the valence e– (i. e. , the ones involved in bonding). C N S octet rule: atoms “want” 8 v. e– -- several exceptions (a topic for later) Gilbert Lewis (1875– 1946)
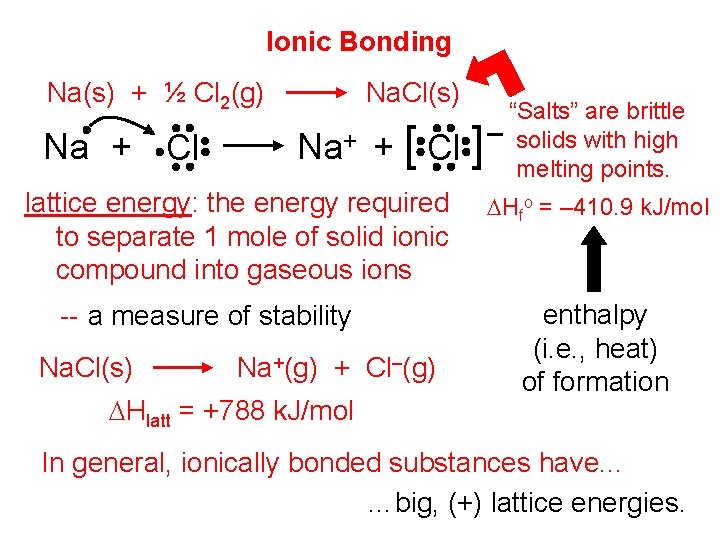
Ionic Bonding Na(s) + ½ Cl 2(g) Na + Cl Na. Cl(s) Na+ + [ Cl ] lattice energy: the energy required to separate 1 mole of solid ionic compound into gaseous ions -- a measure of stability Na. Cl(s) Na+(g) + Cl–(g) DHlatt = +788 k. J/mol “Salts” are brittle – solids with high melting points. DHfo = – 410. 9 k. J/mol enthalpy (i. e. , heat) of formation In general, ionically bonded substances have. . . …big, (+) lattice energies.

Because lattice energies are electrostatic in nature, two variables are involved in how big they are: 1. the magnitude of the charges (“wears the pants”) 2. the separation between the ions Charge can easily be twice as large (i. e. , 2+ and 2– vs. 1+ and 1–), but the separation never varies by that much. Put the following in order of increasing lattice energy: Li. Br, Fe. N, Cd. O. Li. Br < Cd. O < Fe. N Now these: Mg. S, Mg. Cl 2, Mg. O. Mg. Cl 2 < Mg. S < Mg. O
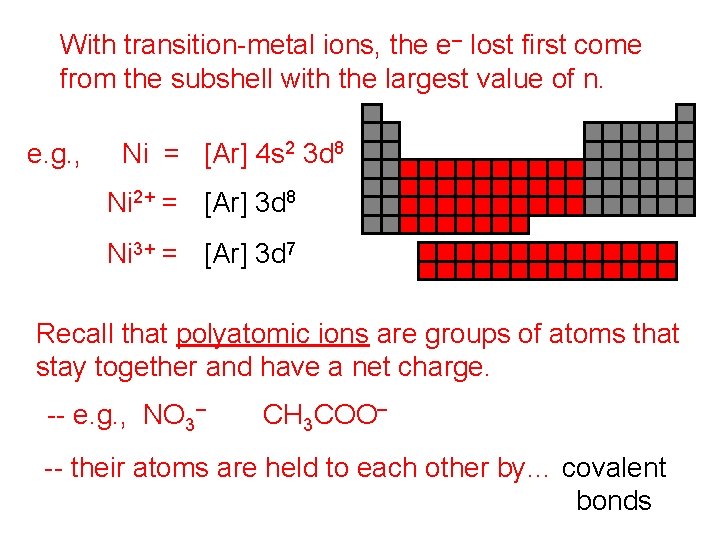
With transition-metal ions, the e– lost first come from the subshell with the largest value of n. e. g. , Ni = [Ar] 4 s 2 3 d 8 Ni 2+ = [Ar] 3 d 8 Ni 3+ = [Ar] 3 d 7 Recall that polyatomic ions are groups of atoms that stay together and have a net charge. -- e. g. , NO 3– CH 3 COO– -- their atoms are held to each other by… covalent bonds
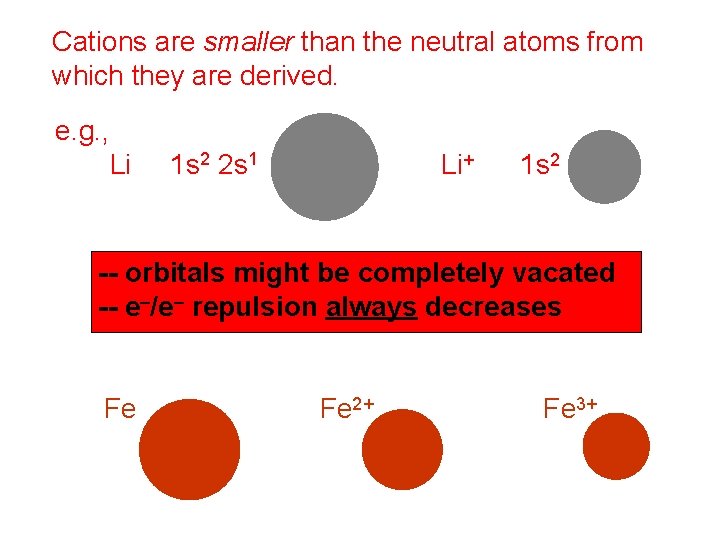
Cations are smaller than the neutral atoms from which they are derived. e. g. , Li 1 s 2 2 s 1 Li+ 1 s 2 -- orbitals might be completely vacated -- e–/e– repulsion always decreases Fe Fe 2+ Fe 3+
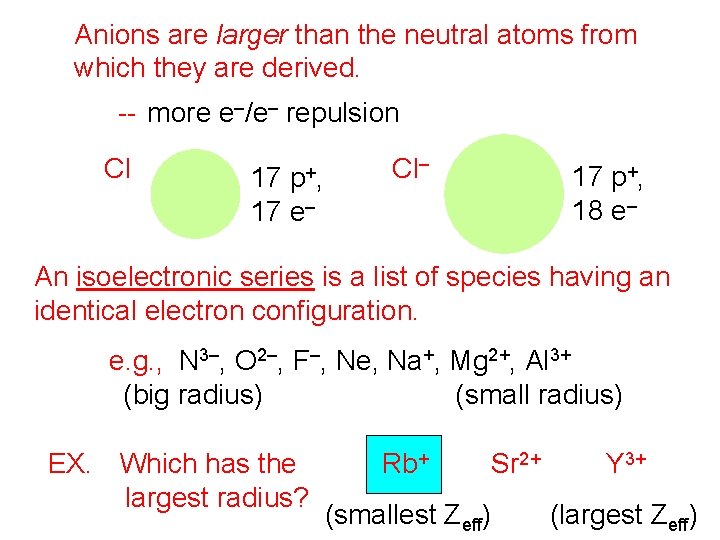
Anions are larger than the neutral atoms from which they are derived. -- more e–/e– repulsion Cl p +, 17 17 e– Cl– 17 p+, 18 e– An isoelectronic series is a list of species having an identical electron configuration. e. g. , N 3–, O 2–, F–, Ne, Na+, Mg 2+, Al 3+ (big radius) (small radius) EX. Which has the largest radius? Rb+ Sr 2+ (smallest Zeff) Y 3+ (largest Zeff)
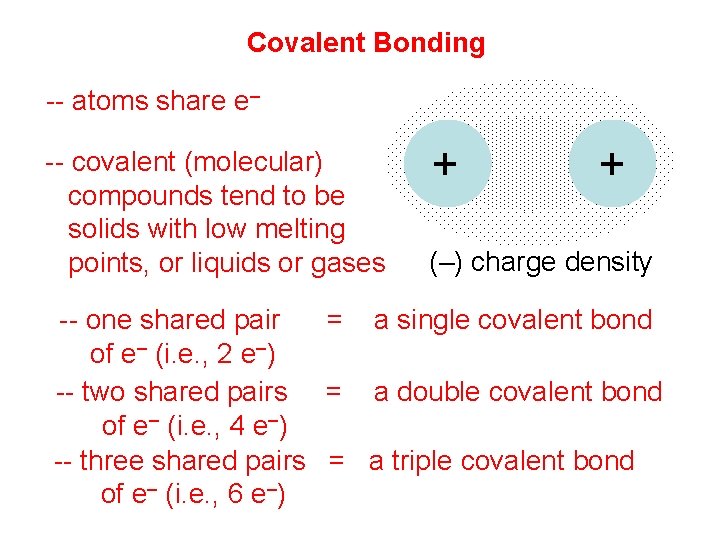
Covalent Bonding -- atoms share e– -- covalent (molecular) compounds tend to be solids with low melting points, or liquids or gases + + (–) charge density -- one shared pair = a single covalent bond of e– (i. e. , 2 e–) -- two shared pairs = a double covalent bond of e– (i. e. , 4 e–) -- three shared pairs = a triple covalent bond of e– (i. e. , 6 e–)
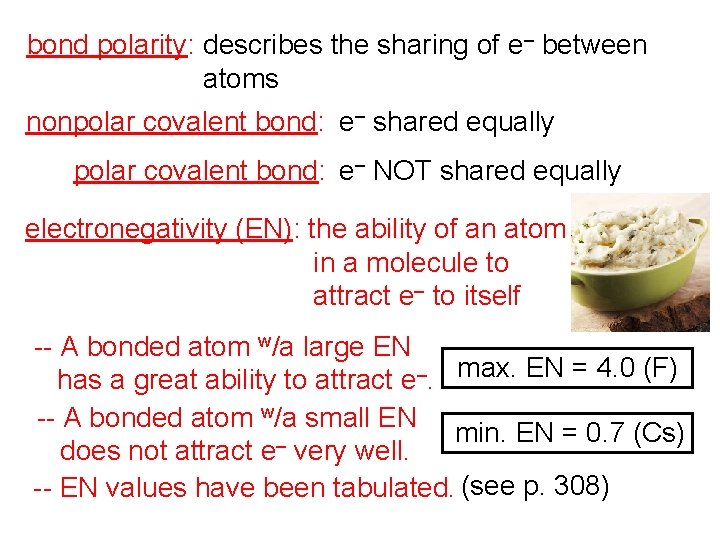
bond polarity: describes the sharing of e– between atoms nonpolar covalent bond: e– shared equally polar covalent bond: e– NOT shared equally electronegativity (EN): the ability of an atom in a molecule to attract e– to itself -- A bonded atom w/a large EN has a great ability to attract e–. max. EN = 4. 0 (F) -- A bonded atom w/a small EN min. EN = 0. 7 (Cs) – does not attract e very well. -- EN values have been tabulated. (see p. 308)

The DEN between bonded atoms approximates the type of bond between them. DEN < 0. 5 < DEN < 2. 0 DEN > 2. 0 nonpolar covalent ionic bond As DEN increases, bond polarity. . . increases. DEN for a C–H bond = 0. 4, so all C–H bonds (such as those in candle wax) are nonpolar.
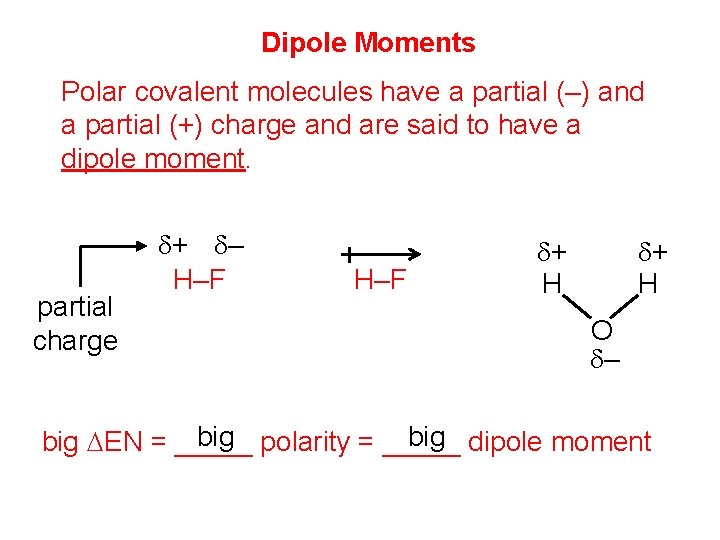
Dipole Moments Polar covalent molecules have a partial (–) and a partial (+) charge and are said to have a dipole moment. partial charge d+ d– H–F d+ H O d– big polarity = _____ big dipole moment big DEN = _____
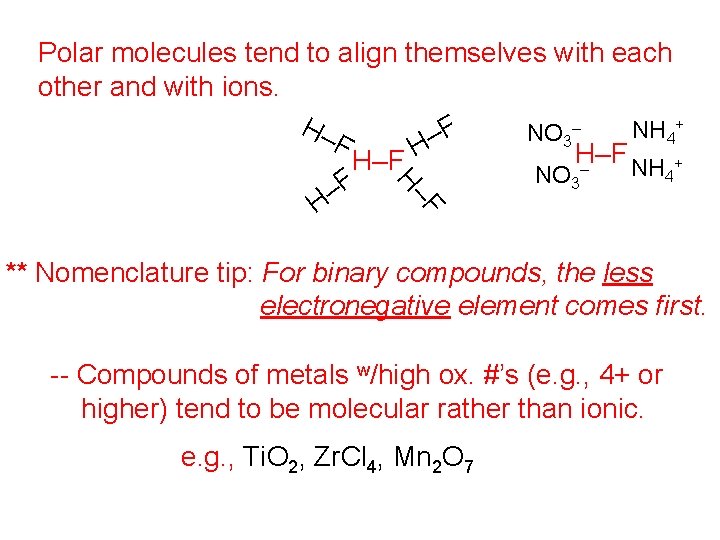
F H– Polar molecules tend to align themselves with each other and with ions. + H– – NH F NO 4 – 3 F H H–F – NH 4+ NO 3 F – H ** Nomenclature tip: For binary compounds, the less electronegative element comes first. -- Compounds of metals w/high ox. #’s (e. g. , 4+ or higher) tend to be molecular rather than ionic. e. g. , Ti. O 2, Zr. Cl 4, Mn 2 O 7
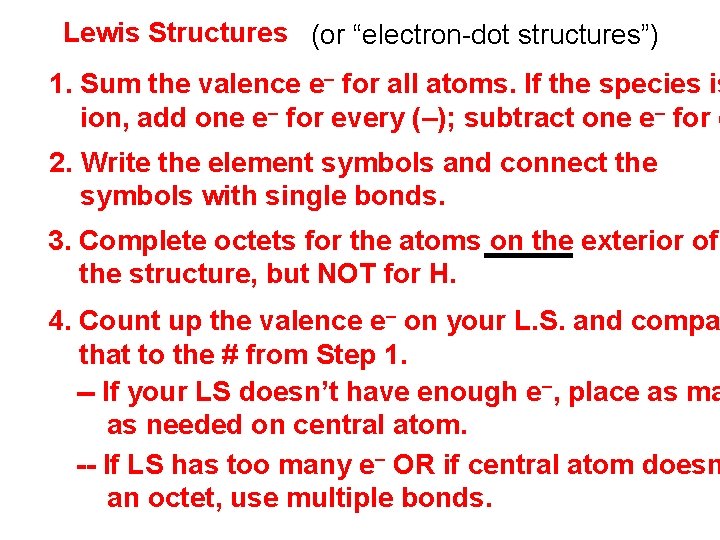
Lewis Structures (or “electron-dot structures”) 1. Sum the valence e– for all atoms. If the species is ion, add one e– for every (–); subtract one e– for e 2. Write the element symbols and connect the symbols with single bonds. 3. Complete octets for the atoms on the exterior of the structure, but NOT for H. 4. Count up the valence e– on your L. S. and compa that to the # from Step 1. -- If your LS doesn’t have enough e–, place as ma as needed on central atom. -- If LS has too many e– OR if central atom doesn an octet, use multiple bonds.
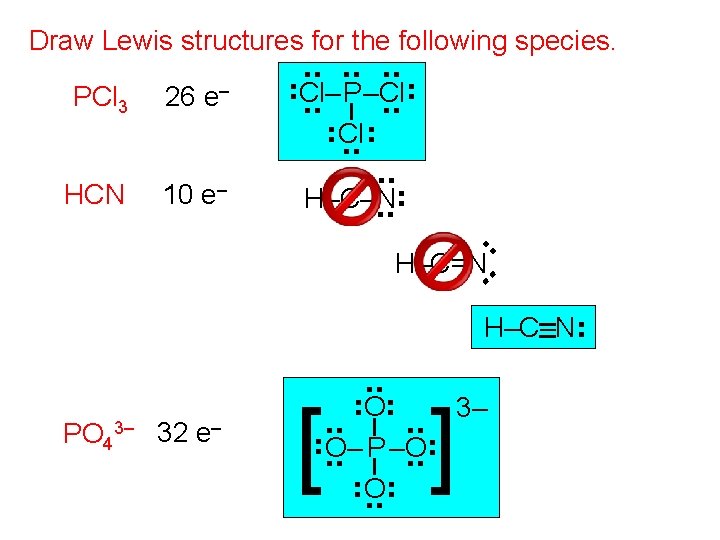
Draw Lewis structures for the following species. 10 e– . . HCN . . . 26 e– . . PCl 3 . . . Cl–. . P–Cl. . . . H–C–N. . H–C=N. . H–C–N . . ] . . . [ O. . O– P –O. . . . PO 43– 32 e– . . 3–
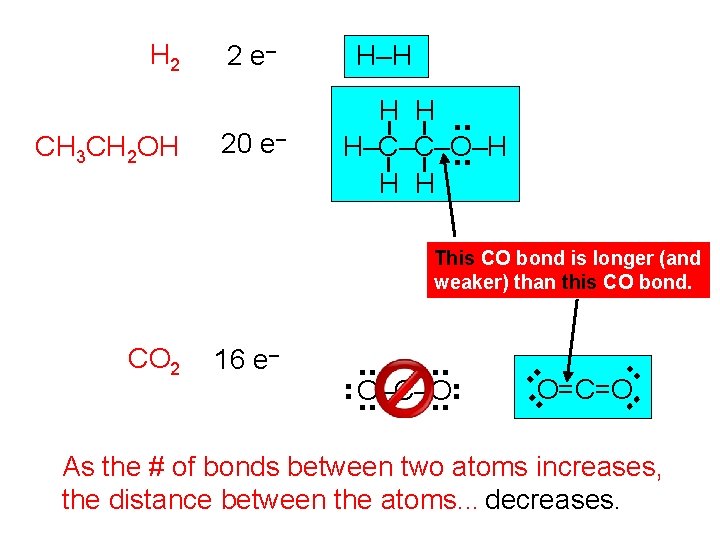
H 2 CH 3 CH 2 OH 2 e– 20 e– H–H H H. . H–C–C–O–H. . H H This CO bond is longer (and weaker) than this CO bond. . . O=C=O. . O–C–O. . . 16 e– . . CO 2 As the # of bonds between two atoms increases, the distance between the atoms. . . decreases.
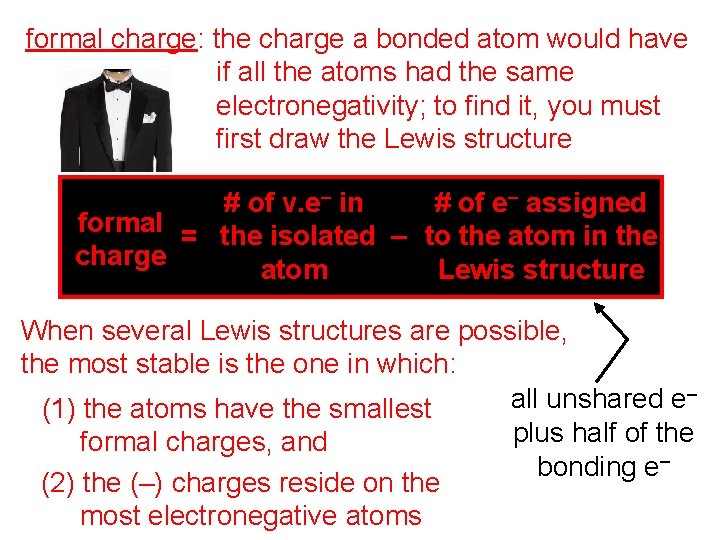
formal charge: the charge a bonded atom would have if all the atoms had the same electronegativity; to find it, you must first draw the Lewis structure # of v. e– in # of e– assigned formal = the isolated – to the atom in the charge atom Lewis structure When several Lewis structures are possible, the most stable is the one in which: – all unshared e (1) the atoms have the smallest plus half of the formal charges, and bonding e– (2) the (–) charges reside on the most electronegative atoms
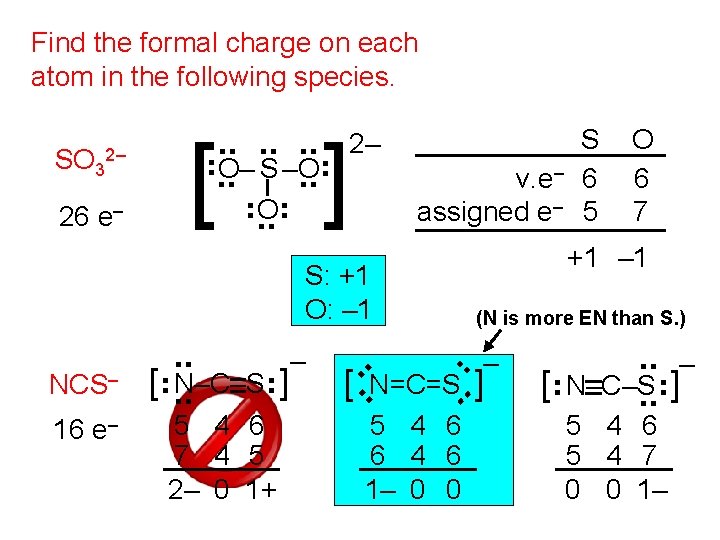
Find the formal charge on each atom in the following species. S v. e– 6 assigned e– 5 S: +1 O: – 1 16 e– 5 4 6 7 4 5 2– 0 1+ +1 – 1 (N is more EN than S. ) . . – [ N=C=S. . ] . . N–C–S . . [ . . NCS– – 5 4 6 6 4 6 1– 0 0 O 6 7 [ – N–C–S ] 5 4 6 5 4 7 0 0 1– . . 2– . . ] . . e– . . 26 [ . . . O– S –O. . . SO 3 2–
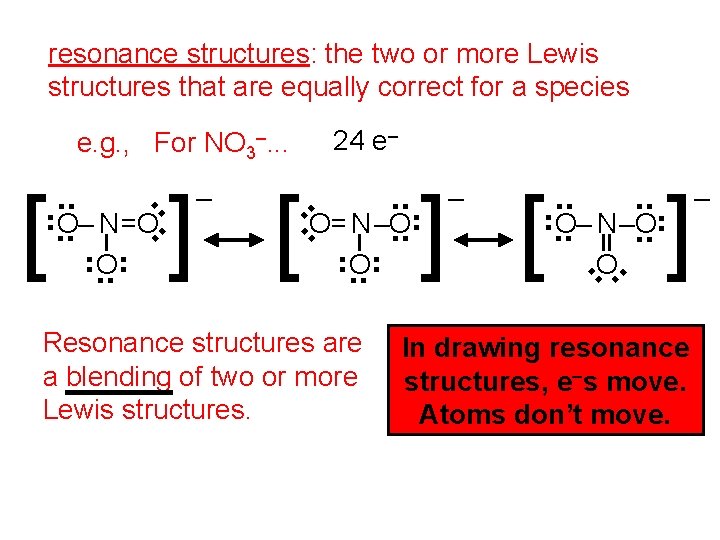
resonance structures: the two or more Lewis structures that are equally correct for a species 24 e– Resonance structures are a blending of two or more Lewis structures. ] [ . . O– N–O. . ] . . – O . . . . O= N–O. . . . – . . ] [ . . O– N=O. . . . e. g. , For NO 3–. . . In drawing resonance structures, e–s move. Atoms don’t move. –
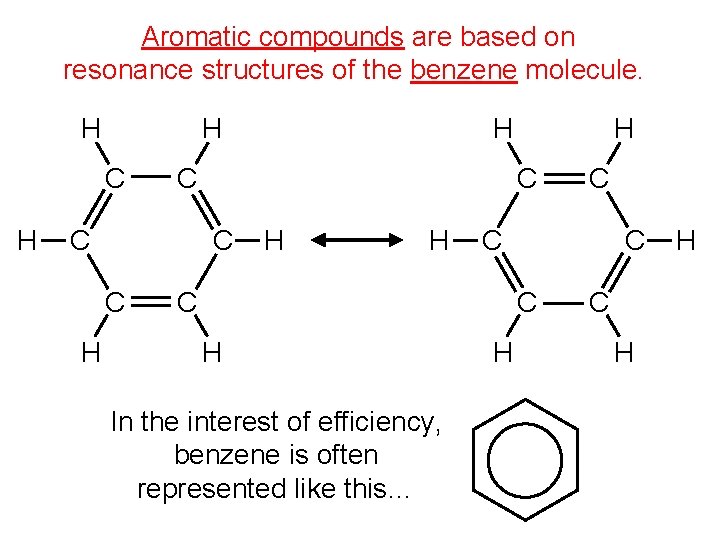
Aromatic compounds are based on resonance structures of the benzene molecule. H H C C C H H H C C H In the interest of efficiency, benzene is often represented like this… C H H
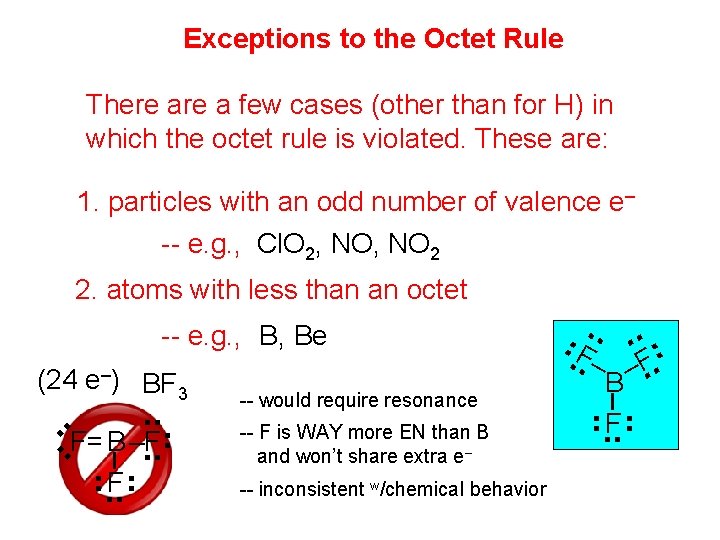
Exceptions to the Octet Rule There a few cases (other than for H) in which the octet rule is violated. These are: 1. particles with an odd number of valence e– -- e. g. , Cl. O 2, NO 2 (24 e–) BF 3 . . . F= B –F. . F . . . . F. – –F. . B -- would require resonance -- F is WAY more EN than B and won’t share extra e– -- inconsistent w/chemical behavior . . F . . -- e. g. , B, Be . . 2. atoms with less than an octet
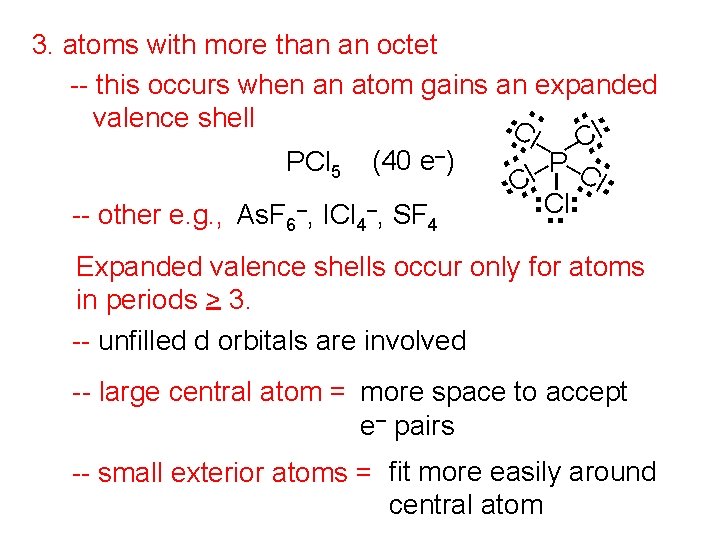
. . . 3. atoms with more than an octet -- this occurs when an atom gains an expanded. . valence shell C. l– C. l. . –. . PCl 5 (40 e ) – P C – l. C. . . l Cl. . -- other e. g. , As. F 6–, ICl 4–, SF 4 Expanded valence shells occur only for atoms in periods > 3. -- unfilled d orbitals are involved -- large central atom = more space to accept e– pairs -- small exterior atoms = fit more easily around central atom
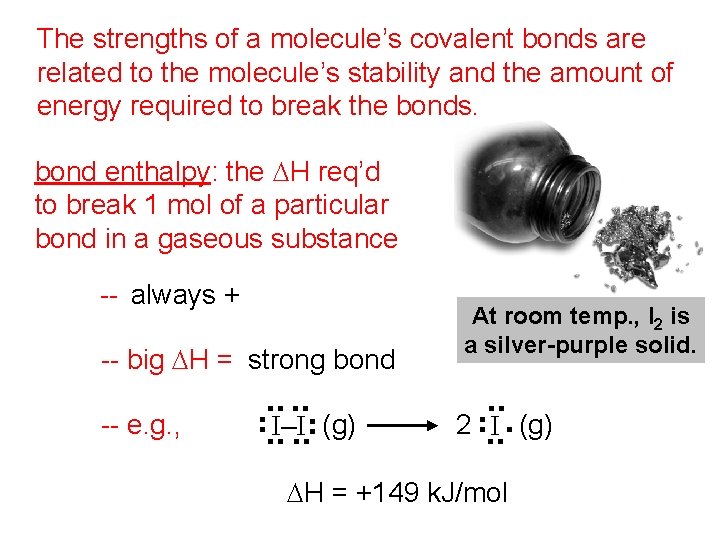
The strengths of a molecule’s covalent bonds are related to the molecule’s stability and the amount of energy required to break the bonds. bond enthalpy: the DH req’d to break 1 mol of a particular bond in a gaseous substance -- big DH = strong bond (g) . . -- e. g. , . . . I–I. . At room temp. , I 2 is a silver-purple solid. 2 . . I (g) . . . -- always + DH = +149 k. J/mol
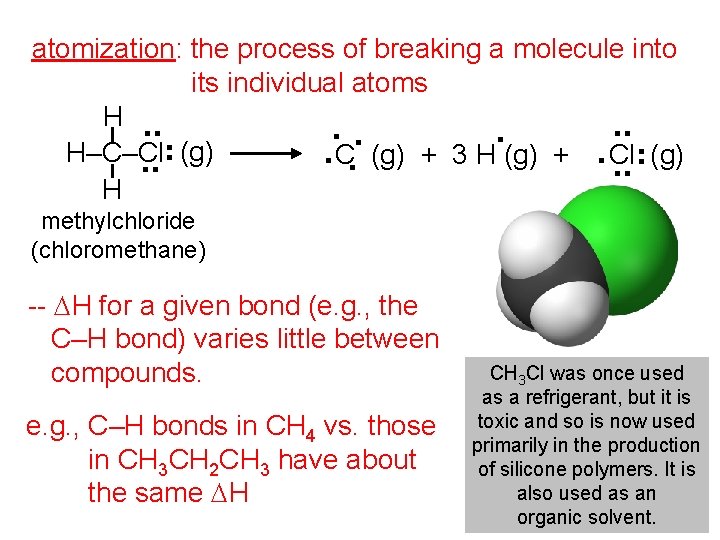
. . atomization: the process of breaking a molecule into its individual atoms H. . H–C–Cl C (g) + 3 H (g) + Cl. . (g) H methylchloride (chloromethane) -- DH for a given bond (e. g. , the C–H bond) varies little between compounds. e. g. , C–H bonds in CH 4 vs. those in CH 3 CH 2 CH 3 have about the same DH CH 3 Cl was once used as a refrigerant, but it is toxic and so is now used primarily in the production of silicone polymers. It is also used as an organic solvent.
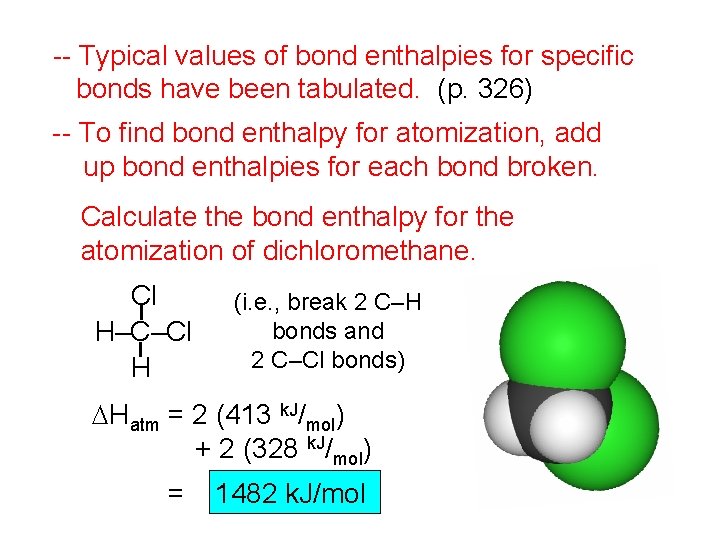
-- Typical values of bond enthalpies for specific bonds have been tabulated. (p. 326) -- To find bond enthalpy for atomization, add up bond enthalpies for each bond broken. Calculate the bond enthalpy for the atomization of dichloromethane. Cl H–C–Cl H (i. e. , break 2 C–H bonds and 2 C–Cl bonds) DHatm = 2 (413 k. J/mol) + 2 (328 k. J/mol) = 1482 k. J/mol
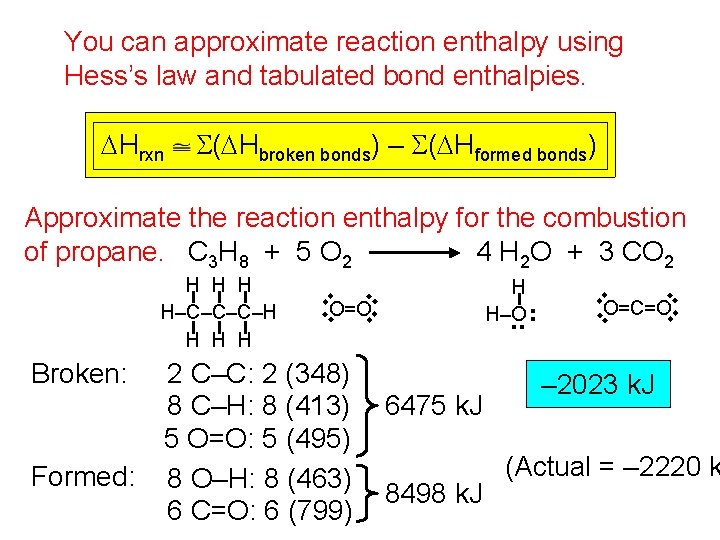
You can approximate reaction enthalpy using Hess’s law and tabulated bond enthalpies. DHrxn S(DHbroken bonds) – S(DHformed bonds) 6475 k. J 8498 k. J . . . 2 C–C: 2 (348) 8 C–H: 8 (413) 5 O=O: 5 (495) 8 O–H: 8 (463) 6 C=O: 6 (799) H H–O . . Formed: . . Broken: O=O . . H H–C–C–C–H H . . Approximate the reaction enthalpy for the combustion of propane. C 3 H 8 + 5 O 2 4 H 2 O + 3 CO 2 O=C=O – 2023 k. J (Actual = – 2220 k
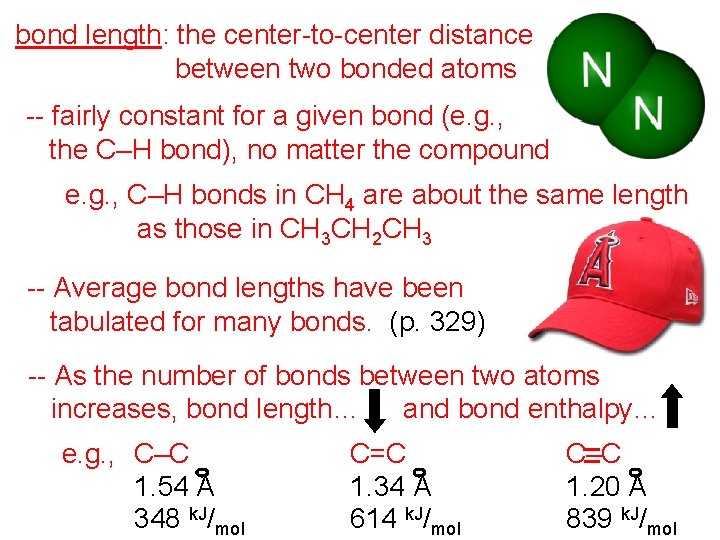
bond length: the center-to-center distance between two bonded atoms -- fairly constant for a given bond (e. g. , the C–H bond), no matter the compound e. g. , C–H bonds in CH 4 are about the same length as those in CH 3 CH 2 CH 3 -- Average bond lengths have been tabulated for many bonds. (p. 329) -- As the number of bonds between two atoms increases, bond length… and bond enthalpy… e. g. , C–C 1. 54 A 348 k. J/mol C=C 1. 34 A 614 k. J/mol C–C 1. 20 A 839 k. J/mol
- Slides: 27