AP Biology Membrane Structure Molecule Transport Part 2
AP Biology Membrane Structure & Molecule Transport Part 2

Cell Membrane

Concept 7. 2: Membrane structure results in selective permeability • A cell must exchange materials with its surroundings, a process controlled by the plasma membrane which acts like a BORDER. • Plasma membranes are made out of a PHOSPHOLIPID bilayer and is selectively permeable, regulating the cell’s molecular traffic. • This ability allows for HOMEOSTASIS of each cell. © 2011 Pearson Education, Inc.
Semi-Permeability • How do cells maintain different concentrations of PROTEINS & MOLECULES on each side of the membrane? They are SEMIPERMEABLE & can control what passes through this border.

Movement • There are many ways substances can move into and out of the cell: – Some simply DRIFT in & out (no cell energy required) – Others require specific types of PROTEINS & a boost of ENERGY

Material Transport • CO 2 and O 2 (both gases) diffuse across wet bilayer because they are neutrally charged particles. • Ions & water move through the proteins because of particle charge “transport proteins”
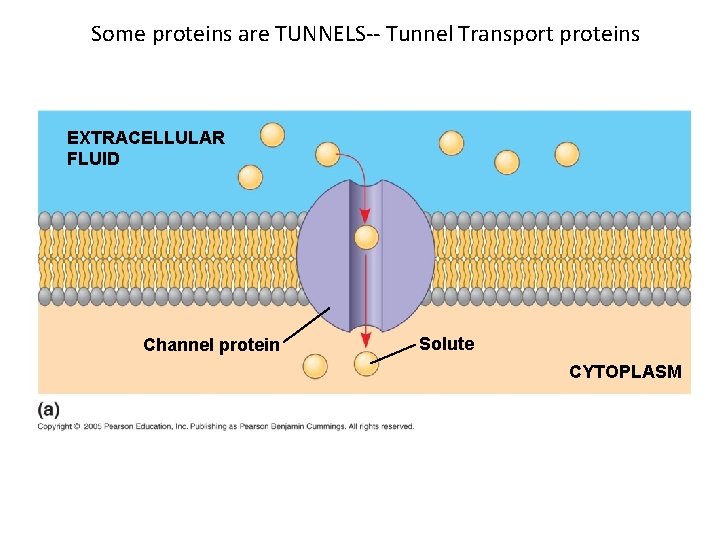
Some proteins are TUNNELS-- Tunnel Transport proteins EXTRACELLULAR FLUID Channel protein Solute CYTOPLASM
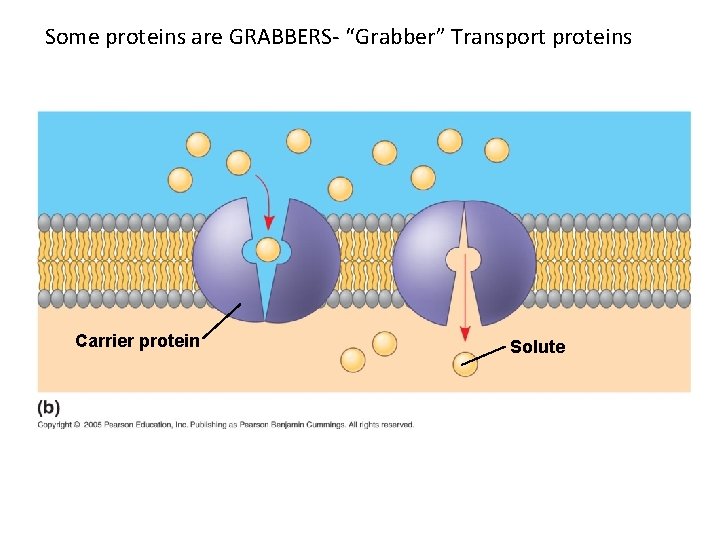
Some proteins are GRABBERS- “Grabber” Transport proteins Carrier protein Solute
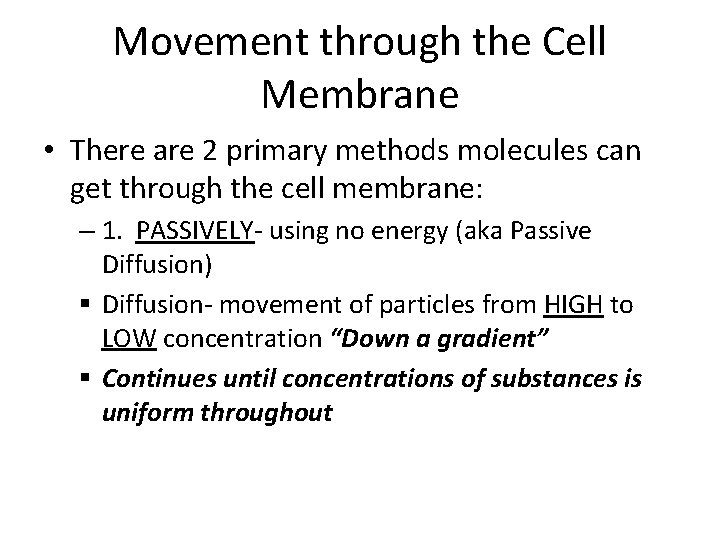
Movement through the Cell Membrane • There are 2 primary methods molecules can get through the cell membrane: – 1. PASSIVELY- using no energy (aka Passive Diffusion) § Diffusion- movement of particles from HIGH to LOW concentration “Down a gradient” § Continues until concentrations of substances is uniform throughout
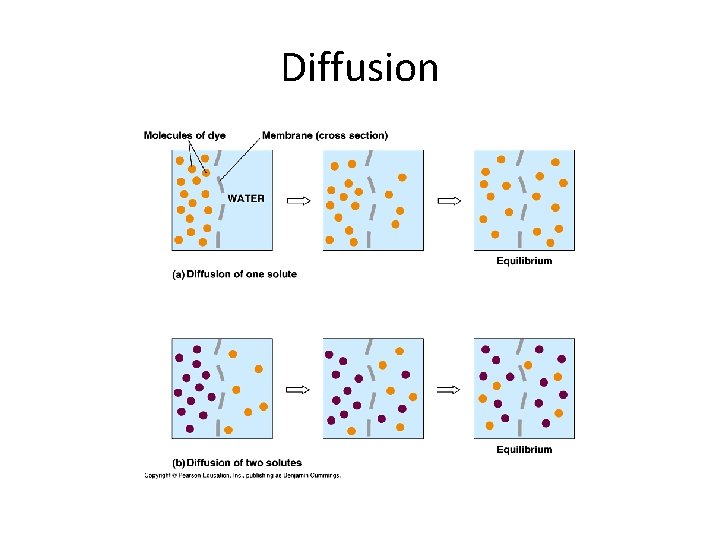
Diffusion
Diffusion • Examples of diffusion in Biology: – Gas exchange in alveoli (in lungs) – Gas exchange in photosynthesis • High diffusion rates will result from: – Short distances – Large surface areas – Large concentration differences

Osmosis • Osmosis= DIFFUSION of WATER through a partially permeable membrane from a more DILUTE solution (hypotonic) to a more CONCENTRATED solution (hypertonic) until it is equal on both sides (isotonic) provided there is no pressure being applied. • Example= absorption of water by plant roots
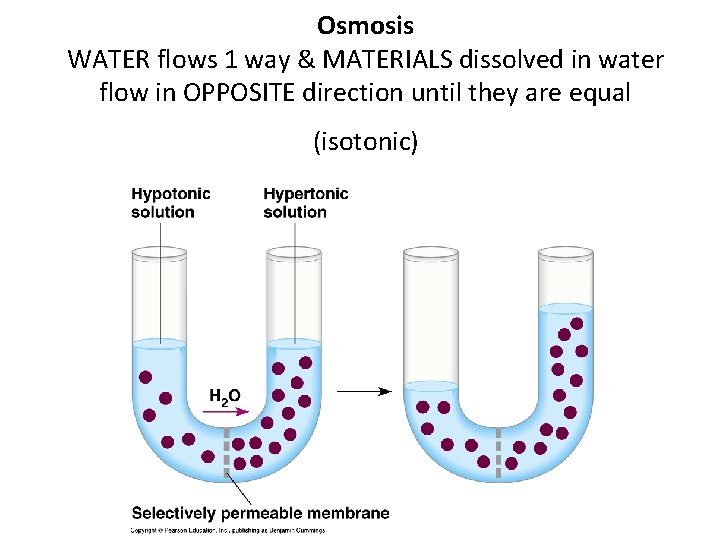
Osmosis WATER flows 1 way & MATERIALS dissolved in water flow in OPPOSITE direction until they are equal (isotonic)

Solutions- Iso/Hypertonic • The prefix refers to the concentration of the SOLUTE dissolved in the water – Iso= SAME – Hypo= less/under; “dilute” solution – Hyper= more/over; “concentrated” solution
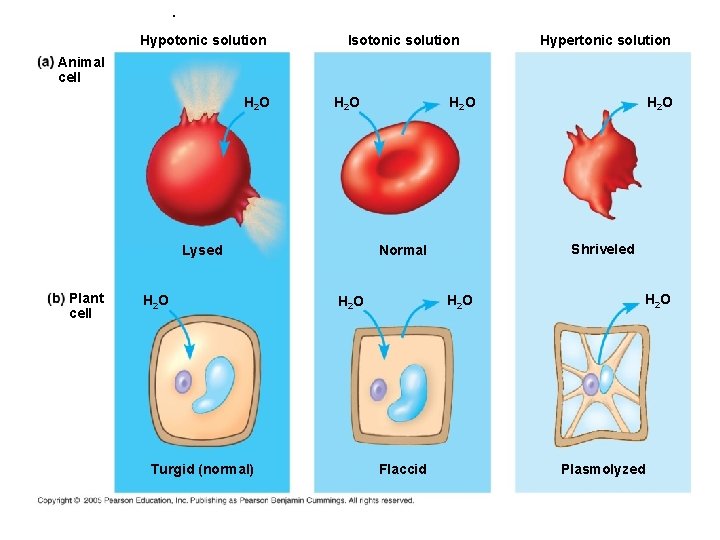
. Hypotonic solution Isotonic solution Hypertonic solution Animal cell H 2 O Turgid (normal) H 2 O Flaccid H 2 O Shriveled Normal Lysed Plant cell H 2 O Plasmolyzed
Osmoregulation= water control • Keeping the concentration of cell cytoplasm or blood at a suitable concentration
Plant Cells • Plant cells remain active in either a HYPOTONIC or HYPERTONIC solution • In a hypotonic solution, cells have a HIGHER water potential or lower TURGOR pressure. This is influenced by the concentration of the SOLUTES
Facilitated Diffusion • Movement of specific molecules DOWN a concentration gradient, passing through the cell membrane via a particular SPECIFIC protein. • Each carrier has its own SHAPE & only allows ONE molecule to pass through • Common molecules entering/ leaving include GLUCOSE & AMINO ACIDS • Passive diffusion is PASSIVE & requires no ENERGY from the cell • Examples include Aquaporins (help move water) & Gated ion channels
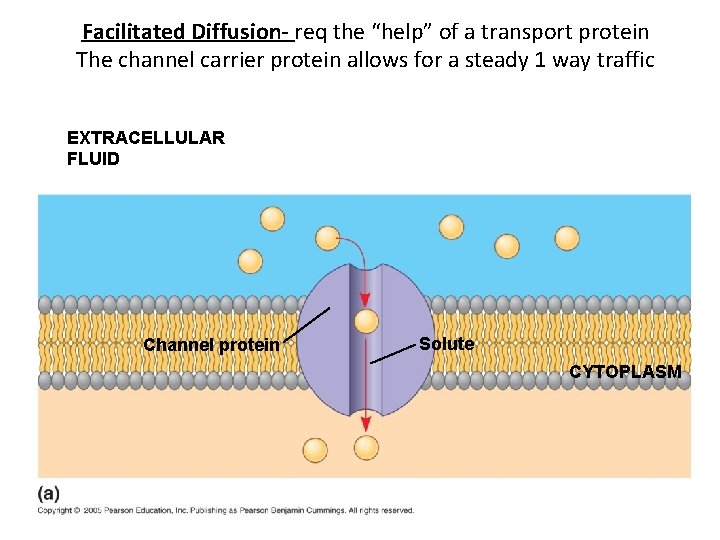
Facilitated Diffusion- req the “help” of a transport protein The channel carrier protein allows for a steady 1 way traffic EXTRACELLULAR FLUID Channel protein Solute CYTOPLASM

Active Transport= requires ENERGY • Movement of a substance across a cell membrane AGAINST its concentration gradient from LOWER concentration to a HIGHER concentration. • Special proteins within the cell membrane act as specific protein ‘carriers’. • The energy for active transport comes from ATP generated by respiration
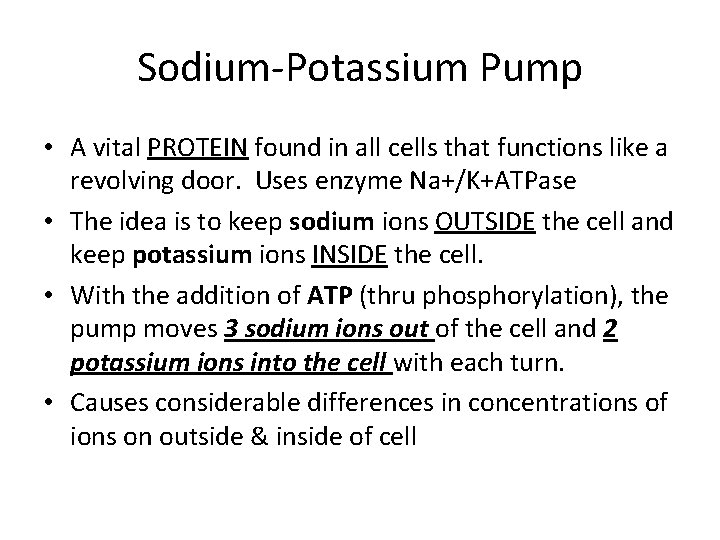
Sodium-Potassium Pump • A vital PROTEIN found in all cells that functions like a revolving door. Uses enzyme Na+/K+ATPase • The idea is to keep sodium ions OUTSIDE the cell and keep potassium ions INSIDE the cell. • With the addition of ATP (thru phosphorylation), the pump moves 3 sodium ions out of the cell and 2 potassium ions into the cell with each turn. • Causes considerable differences in concentrations of ions on outside & inside of cell
![Active Transport EXTRACELLULAR [Na+] high FLUID [K+] low Na+ Na+ CYTOPLASM [Na+] low [K+] Active Transport EXTRACELLULAR [Na+] high FLUID [K+] low Na+ Na+ CYTOPLASM [Na+] low [K+]](http://slidetodoc.com/presentation_image_h/0693cb44a126494d0fc58055e580fde1/image-22.jpg)
Active Transport EXTRACELLULAR [Na+] high FLUID [K+] low Na+ Na+ CYTOPLASM [Na+] low [K+] high Na+ Cytoplasmic Na + bonds to the sodium-potassium pump P ATP P ADP Na+ binding stimulates phosphorylation by ATP. Phosphorylation causes the protein to change its conformation, expelling Na + to the outside. K+ K+ P Extracellular K+ binds to the protein, triggering release of the phosphate group. K+ Loss of the phosphate restores the protein’s original conformation. K+ is released and Na + sites are receptive again; the cycle repeats.

Why is the difference important? • Essential for: – Communication – p. H – Volume – Nutrients • The Sodium-Potassium Pump can be thought of as a magnificent “Molecular Nanomachine” • Example- If Na/K Pump doesn’t function properly in the BRAIN, severe neurological conditions like migraines w/muscle spasms can occur. New meds are being developed to target the pump.
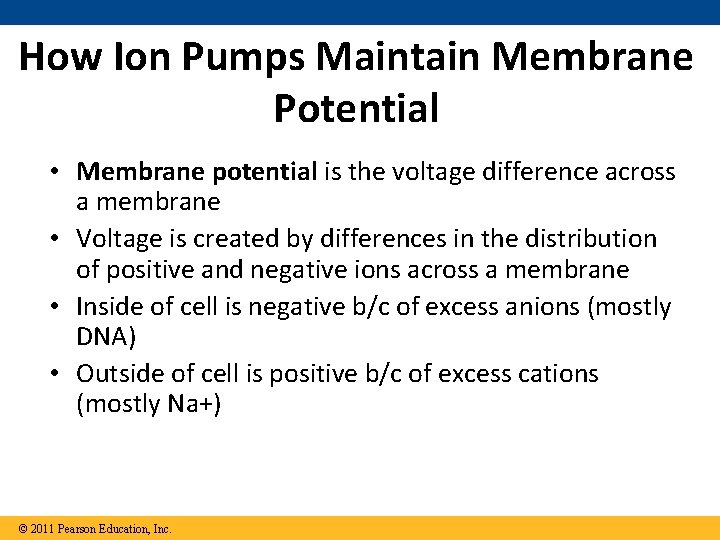
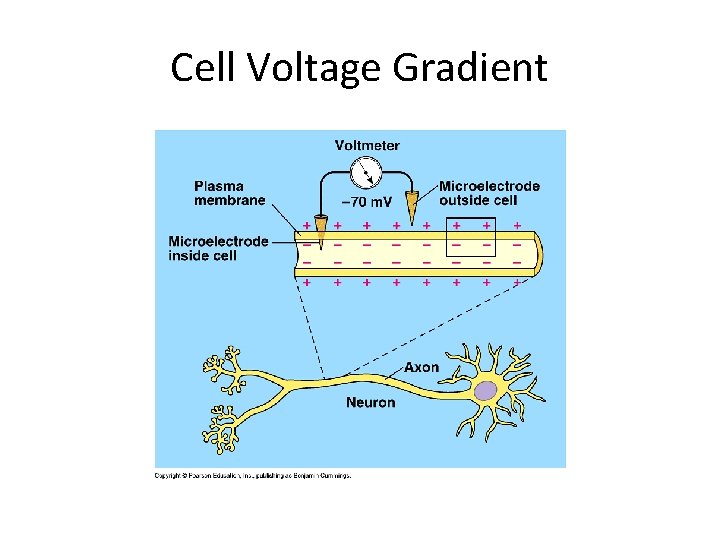
How Ion Pumps Maintain Membrane Potential • Membrane potential is the voltage difference across a membrane • Voltage is created by differences in the distribution of positive and negative ions across a membrane • Inside of cell is negative b/c of excess anions (mostly DNA) • Outside of cell is positive b/c of excess cations (mostly Na+) © 2011 Pearson Education, Inc.

• Two combined forces, collectively called the electrochemical gradient, drive the diffusion of ions across a membrane – A chemical force (the ion’s concentration gradient) – An electrical force (the effect of the membrane potential or charge on the ion’s movement) © 2011 Pearson Education, Inc.
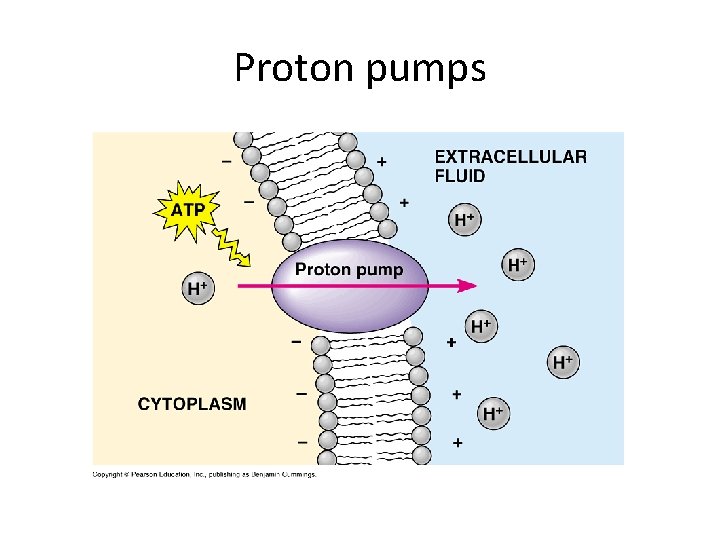
• An electrogenic pump is a transport protein that generates voltage across a membrane- The most important active transport protein for all life forms • The sodium-potassium pump is the major electrogenic pump of animal cells • The main electrogenic pump of plants, fungi, and bacteria is a proton pump • Electrogenic pumps help store energy that can be used for cellular work © 2011 Pearson Education, Inc.
Cell Voltage Gradient
Proton pumps

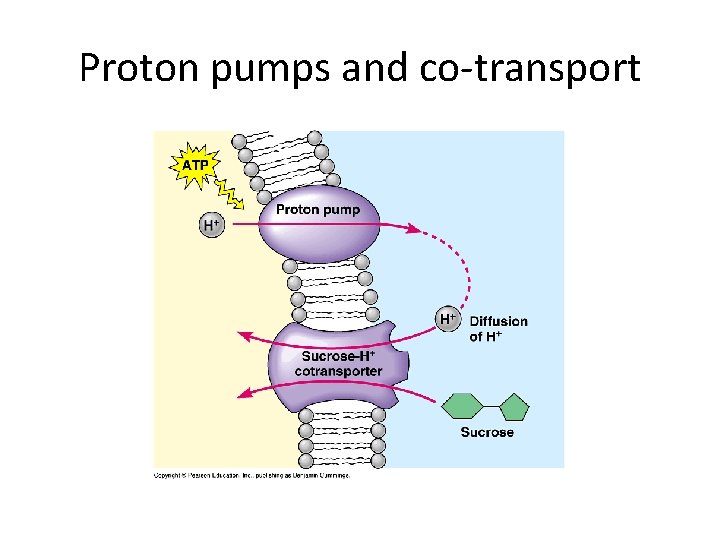
Co-transport • Protons (H+) act like “party invitations” • Require little energy to send them out of cell, then, they bring in “invited” guests only • Important in helping cells of plants & animals be able to take up glucose or sucrose by active transport.
Proton pumps and co-transport
Bulk Transport • The movement of MACROMOLECULES such as PROTEINS or even POLYSACCHARIDES into or out of the cell (transport those molecules TOO big for proteins to transport) • 2 types: – Exocytosis “out” – Endocytosis “in”

Exocytosis “out” • Materials are EXPORTED out of cell using SECRETORY vesicles. • During the process, Golgi complex transforms macromolecules into transport vesicles that move to FUSE with plasma membrane. • Fusion causes vesicle to DUMP its contents out of the cell. • Exocytosis allows cells to get rid of WASTE materials • Examples= 1)pancreas cells releasing hormone insulin into bloodstream to help regulate blood glucose levels. 2) nerve cells releasing neurotransmitters
Endocytosis= “in” • Move materials into the cell through the cell membrane • 3 types: – Pinocytosis “cell drinking” – Phagocytosis “cell eating” – Receptor-mediated – uses ligands
Phagcytosis • Cell’s plasma membrane surrounds a MACROMOLECULE from the EXTRACELLULAR environment and buds off to form a food VACUOLE or phagosome • Phagocytosis is “CELLULAR EATING” • The newly-formed phagosome fuse with a LYSOSOME and digests the food inside.

Pinocytosis • “CELLULAR DRINKING” • Cell ENGULFS substances by PINCHING in and forming vesicles that are smaller than the phagosomes formed in phagocytosis. • Pinocytosis is NON-SPECIFIC and cell takes in whatever solutes are dissolved in the liquid it envelops.
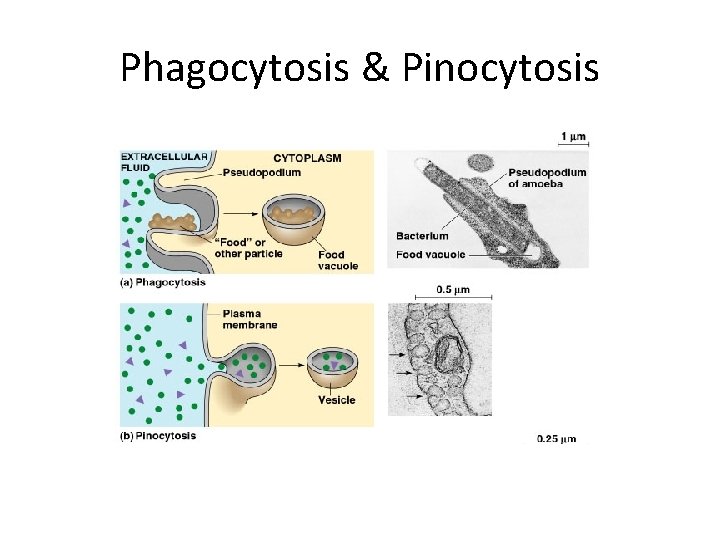
Phagocytosis & Pinocytosis
Receptor-mediated Endocytosis • Extremely SELECTIVE process of importing materials into the cell • SPECIFICITY is controlled by RECEPTOR proteins located on the DEPRESSED areas of the cell membraned called COATED pits. • In receptor-mediated endocytosis, the cell only takes in an extracellular molecule (called a Ligand) if it binds to its specific receptor protein on the cell’s surface.
Receptor-mediated Endocytosis • After the molecule is bound to the cell’s surface, the COATED pit where the receptor protein is located, pinches INWARD to form a coated vesicle. • Similar to phagocytosis, this coated vesicle fuses with a lysosome to DIGEST the engulfed material then releases it into the CYTOSOL.
Example- Familial Hypercholesterolemia • Causes high blood cholesterol • Cholesterol travels in blood as LDL particles • These LDL particles act as ligands to specifically bind to LDL receptor proteins and trigger endocytosis to occur so LDL can be taken in for synthesis of membranes & other steroids • People with this genetic disorder lack the LDL receptor proteins so LDL cannot enter cells and will build up in blood stream causing high cholesterol.

. RECEPTOR-MEDIATED ENDOCYTOSIS Coat protein Receptor Coated vesicle Coated pit Ligand A coated pit and a coated vesicle formed during receptormediated endocytosis (TEMs). Coat protein Plasma membrane 0. 25 µm

Water Potential https: //www. youtube. com/watch? v= n. DZud 2 g 1 RVY

Water Potential • the tendency of water to leave one place in favor of another. Water always moves from an area of higher water potential to an area of lower water potential. • affected by two factors: – pressure – the amount of solute.
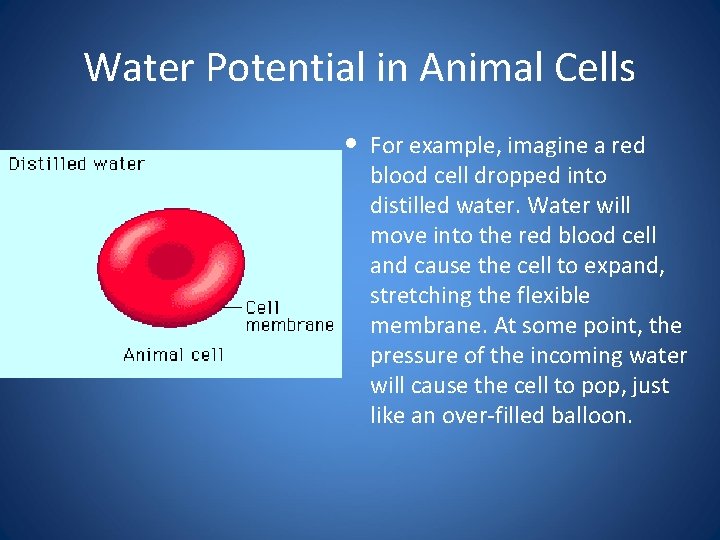
Water Potential in Animal Cells • For example, imagine a red blood cell dropped into distilled water. Water will move into the red blood cell and cause the cell to expand, stretching the flexible membrane. At some point, the pressure of the incoming water will cause the cell to pop, just like an over-filled balloon.

Water Potential in Animal Cells • Why don't red blood cells pop in the bloodstream? • Red blood cells don't pop because the blood provides an isotonic environment for the cells.
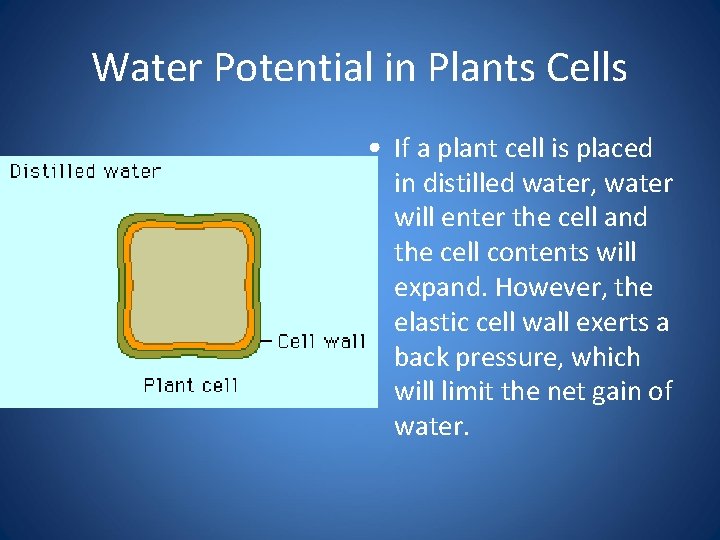
Water Potential in Plants Cells • If a plant cell is placed in distilled water, water will enter the cell and the cell contents will expand. However, the elastic cell wall exerts a back pressure, which will limit the net gain of water.

Calculating Water Potential • Water potential is calculated using the following formula: • Water potential = pressure potential + solute potential

Factors that Affect Water Potential • Pressure potential – Water will move from the area of high pressure to the area of low pressure – In a plant cell, pressure exerted by the rigid cell wall that limits further water uptake. • Solute potential – Water will move from the area of high solute potential (low solute concentration) to the area of lower solute potential (higher solute concentration) – The effect of solute concentration. Pure water at atmospheric pressure has a solute potential of zero. As solute is added, the value for solute potential becomes more negative. This causes water potential to decrease also. In sum, as solute is added, the water potential of a solution drops, and water will tend to move into the solution.
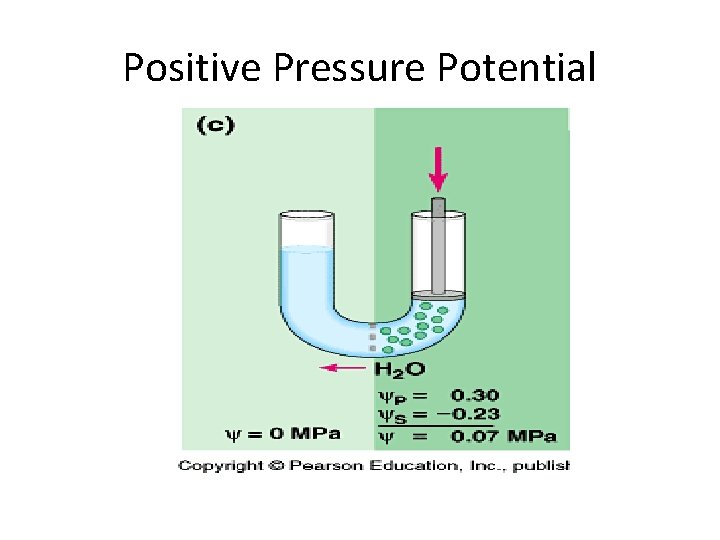
Positive Pressure Potential
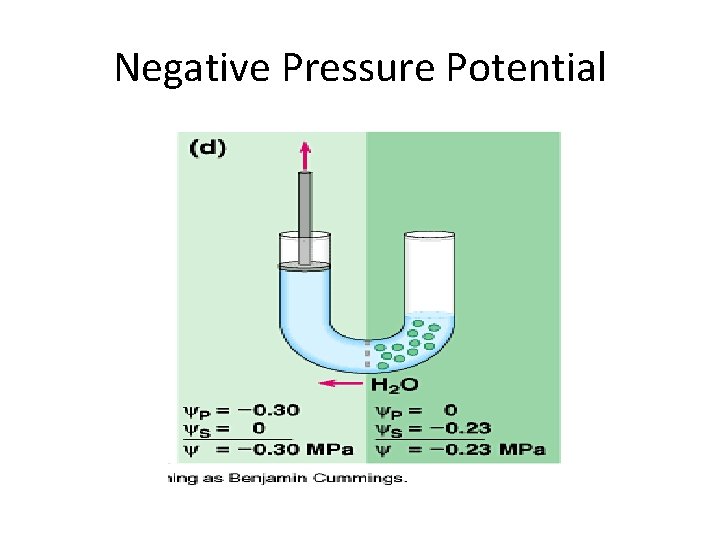
Negative Pressure Potential
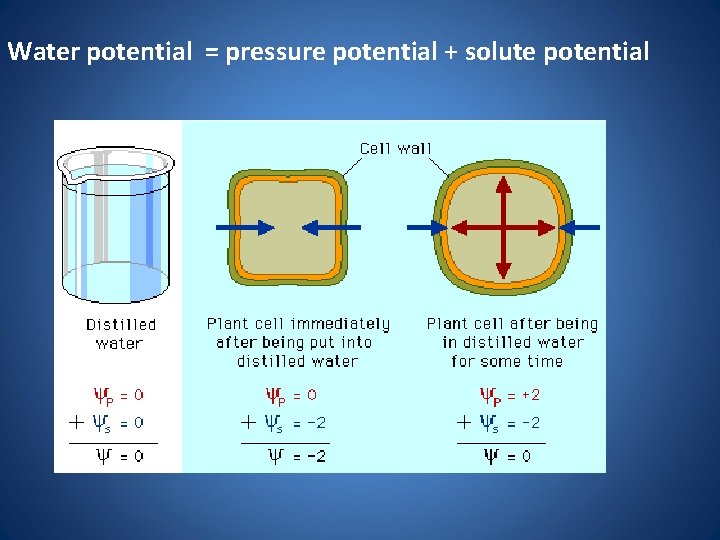
Water potential = pressure potential + solute potential
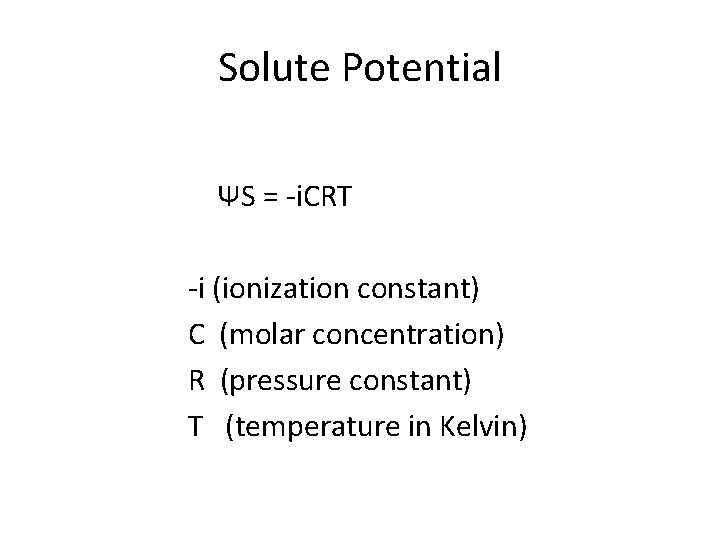
Solute Potential ΨS = -i. CRT -i (ionization constant) C (molar concentration) R (pressure constant) T (temperature in Kelvin)

Ψs= -i. CRT • i - Ionization constant – Greater ionization decreases water potential/increases water movement, OR decrease in ionization increases water potential/decreases water movement. • C – Concentration – Increase in concentration decreases water potential/increases water movement, OR Decrease in concentration increases water potential/decreases water movement • R - Pressure constant – No change in water potential/movement • T – Temperature – Increase in temperature decreases water potential/increases water movement, OR Decrease in temperature increases water potential/decreases water movement.

Ψs= -i. CRT • i - Ionization constant – Usually 1 – 2, inclusively • C – Molar Concentration of Solute – Increase in concentration decreases water potential/increases water movement, OR Decrease in concentration increases water potential/decreases water movement • R - Pressure constant (0. 0831 liters/mole K) – No change in water potential/movement • T – Temperature = in Kelvin (273 + degrees Celsius) – Increase in temperature decreases water potential/increases water movement, OR Decrease in temperature increases water potential/decreases water movement.

Practice • The molar concentration of a sugar solution in an open beaker has been determined to be 0. 2 M. Calculate the solute potential at 22 degrees Celsius. – (1)(. 2)(0. 0831)(295) = -4. 9029 or -5 bars • What is the overall water potential? – Water potential = solute potential + pressure potential; in an open beaker, the pressure potential is 0 so the overall water potential is -5 bars.
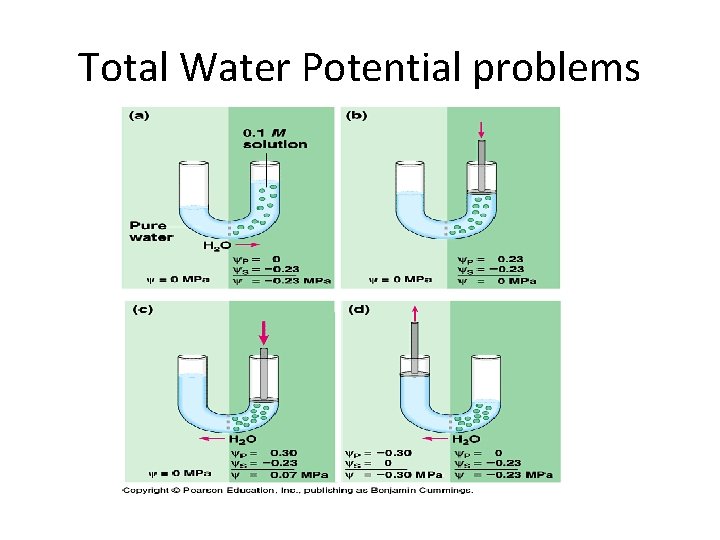
Total Water Potential problems
- Slides: 55