AP BIOLOGY Chapter 4 Carbon Chapter 5 Macromolecules
AP BIOLOGY Chapter 4 Carbon Chapter 5 Macromolecules
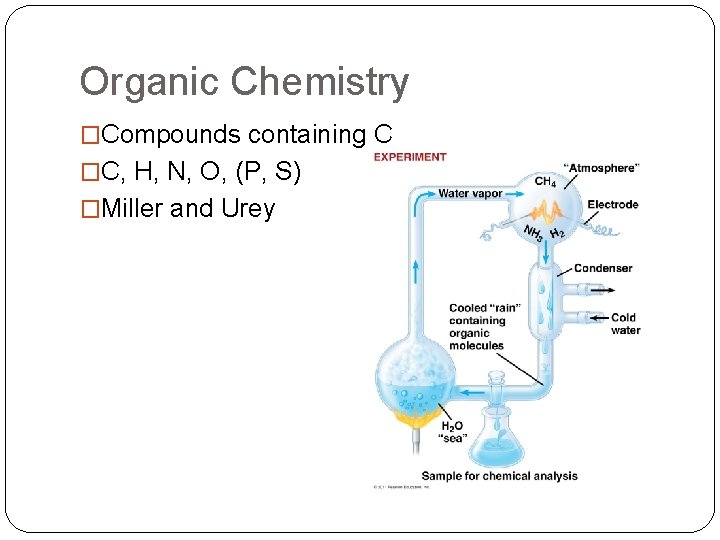
Organic Chemistry �Compounds containing C �C, H, N, O, (P, S) �Miller and Urey
Carbon bond Formation �CH 4 �C 2 H 6 �C 2 H 4
Hydrocarbons �Organic molecules consisting of C and H only �Not prevalent in most living organisms, but most have regions of C and H �Examples: Fats �C-H are nonpolar, (similar electronegativity's) �Their rxs release lots of energy
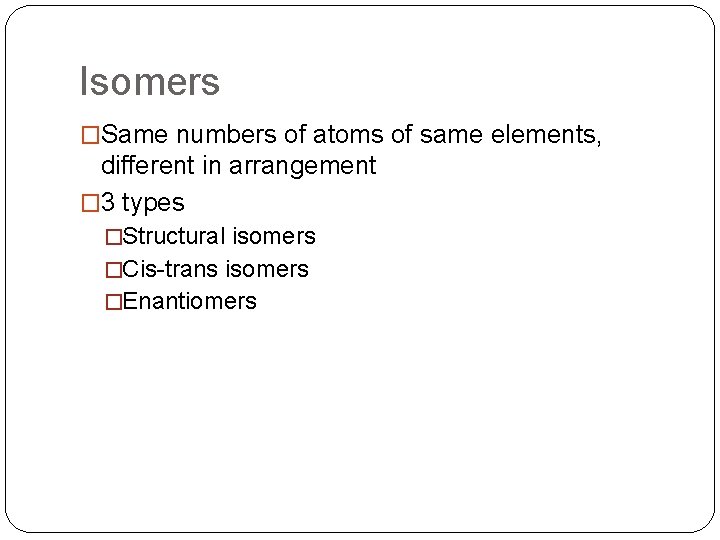
Isomers �Same numbers of atoms of same elements, different in arrangement � 3 types �Structural isomers �Cis-trans isomers �Enantiomers
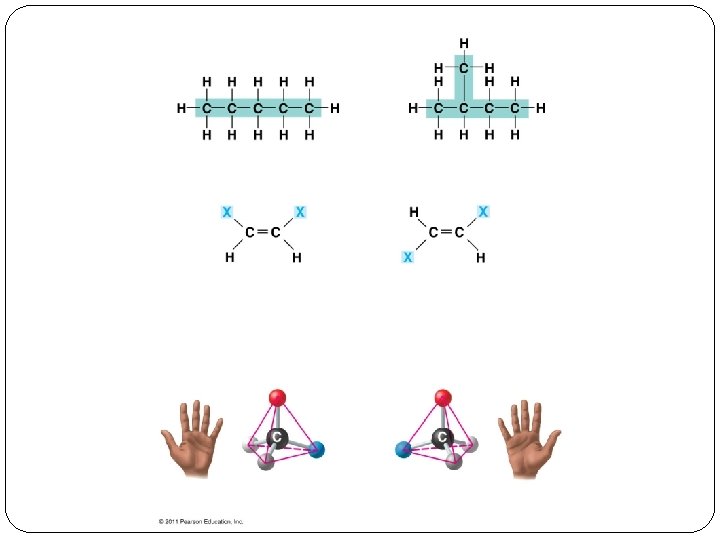
Functional Groups �Groups that are directly involved in chemical reactions Structure Functional Group Example Hydroxyl -OH Alcohols Carbonyl >CO Ketones Aldehydes Carboxyl -COOH Carboxylic acids Amino -NH 2 Amines Sulfhydryl -SH Thiols Phosphate -OPO 32 - Organic phosphates Methyl CH 3 Methylated compounds Drawing
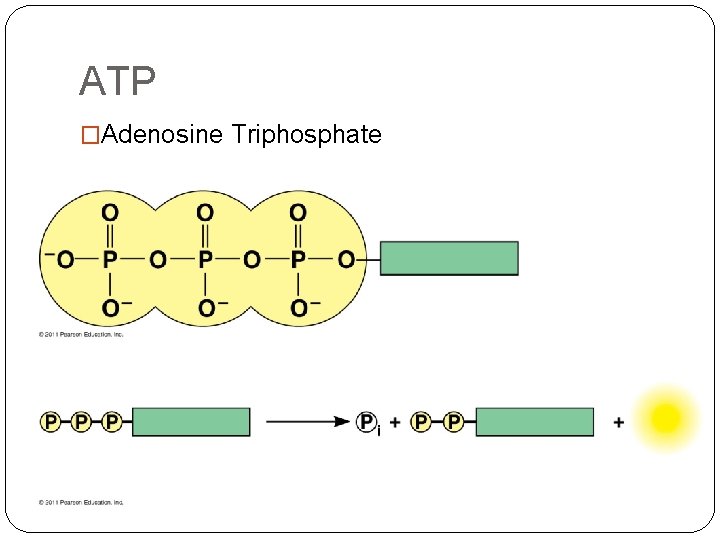
ATP �Adenosine Triphosphate
Macromolecules Chapter 5 �All living things fall under 4 major classes �Carbs �Lipids �Proteins �Nucleic acids 3 of the four are HUGE on a molecular scale (proteins, carbs, nucleic acids)
�Monomer- building blocks of polymers �Polymer- long molecule consisting of many similar or identical building blocks linked by chemical bonds
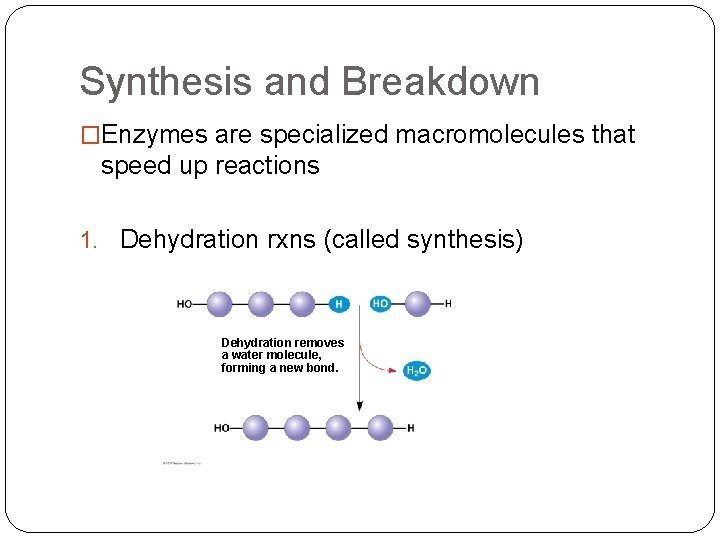
Synthesis and Breakdown �Enzymes are specialized macromolecules that speed up reactions 1. Dehydration rxns (called synthesis) Dehydration removes a water molecule, forming a new bond.
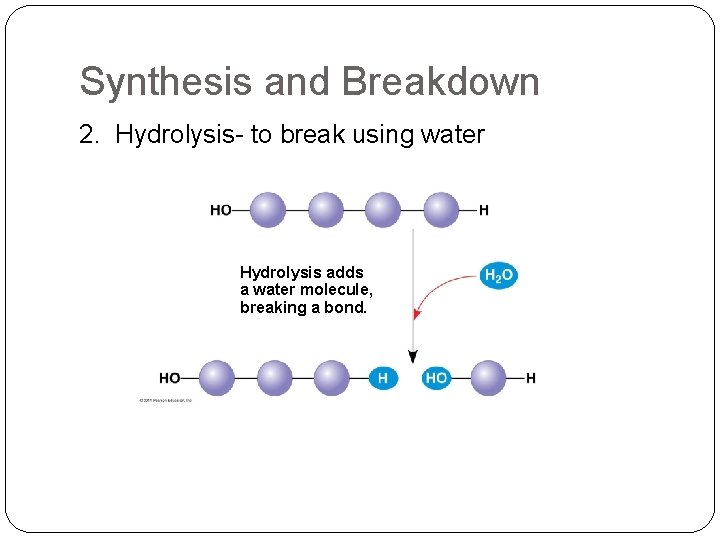
Synthesis and Breakdown 2. Hydrolysis- to break using water Hydrolysis adds a water molecule, breaking a bond.
Carbohydrates �Include both sugars and polymers of sugar �Monosaccharides (CH 2 O) �Molecule has a carbonyl group, and hydroxyl groups �Glucose, fructose, galactose �Disaccharides (2 monosacch. Joined by glycosidic linkage) �Glycosidic linkages are = to dehydration reaction �Maltose, sucrose, lactose
Carbohydrates �Polysaccharides �Joined by glycosidic linkages � 2 major categories of polysaccharides 1. Storage polysaccharides �Starch- plants store as energy within plastids and cholorplasts � Others include: amylose (unbranched) amylopectin �Glycogen- animals store glycogen mainly in liver and muscle cells � Hydrolysis of glycogen releases glucose
Carbohydrates 2. Structural polysaccharides �Cellulose- major component of cell wall in plants �Chitin- used by arthropods to build their exoskeletons �Exoskeleton- hard case that surrounds the soft part of an animal

Proteins �Important in almost everything organisms do �Varied functions �Catalysts- speed up rxns without being consumed in rxn
Proteins �Polypeptides �Monomer- amino acids (all polymers are constructed from same set of 20 amino acids) �Polypeptides- polymers of amino acids �Protein- biologically functional molecule that consists of 1 or more polypeptides, each folded and coiled into a specific structure
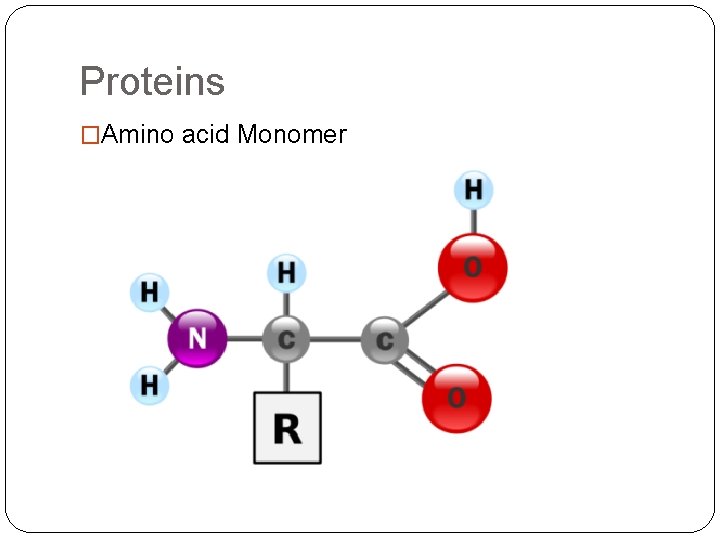
Proteins �Amino acid Monomer
Protein Function 1. Enzymatic-accelerate chemical rxns 2. Defensive- protection against disease 3. Storage 4. Transport- movement across cell membranes 5. Hormonal 6. Receptor- response of cells to chemical stimuli 7. Contractile/motor- movement 8. Structural- support (see page 78 for more detail)
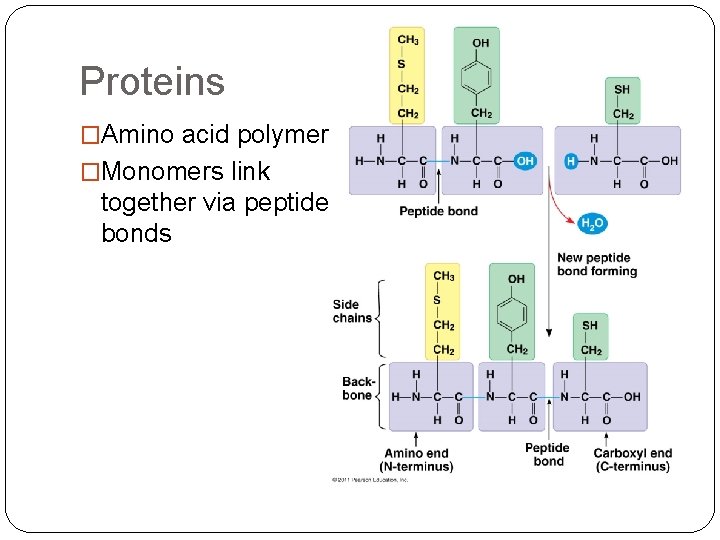
Proteins �Amino acid polymers �Monomers link together via peptide bonds
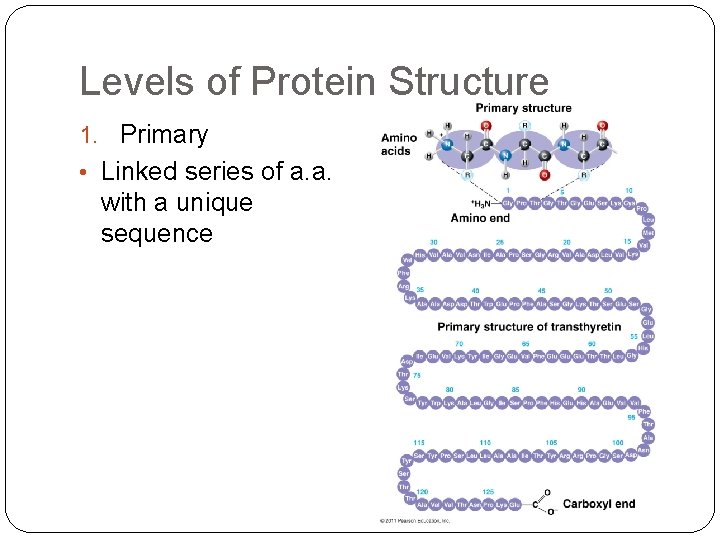
Levels of Protein Structure 1. Primary • Linked series of a. a. with a unique sequence
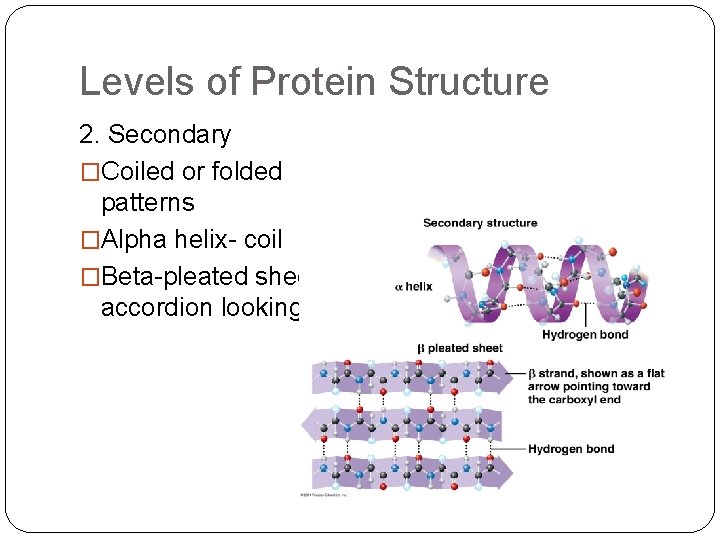
Levels of Protein Structure 2. Secondary �Coiled or folded patterns �Alpha helix- coil �Beta-pleated sheetaccordion looking
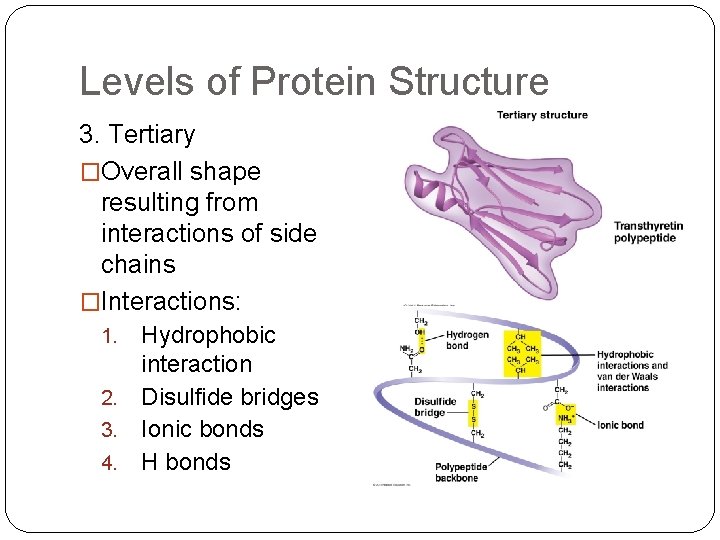
Levels of Protein Structure 3. Tertiary �Overall shape resulting from interactions of side chains �Interactions: Hydrophobic interaction 2. Disulfide bridges 3. Ionic bonds 4. H bonds 1.
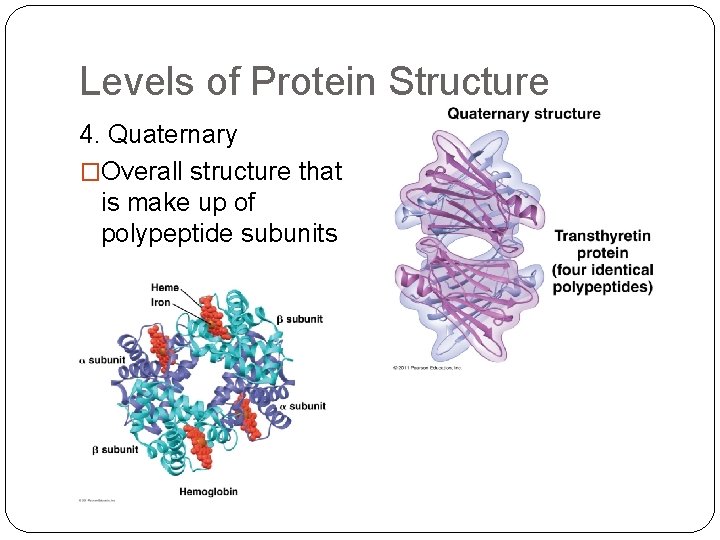
Levels of Protein Structure 4. Quaternary �Overall structure that is make up of polypeptide subunits
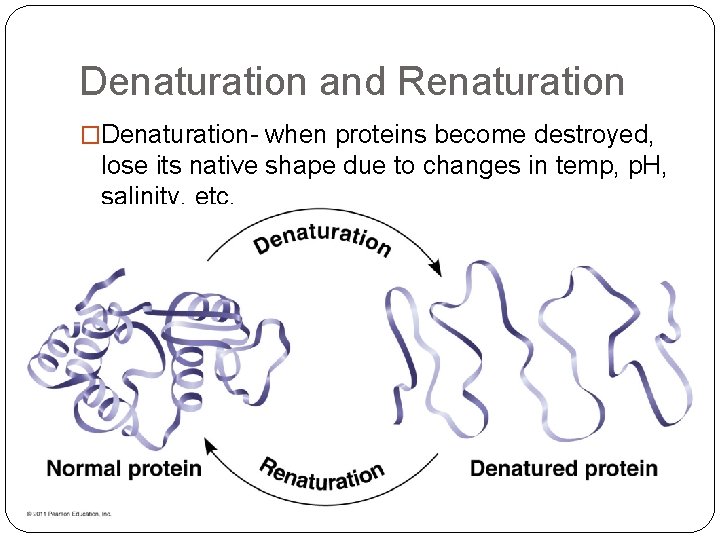
Denaturation and Renaturation �Denaturation- when proteins become destroyed, lose its native shape due to changes in temp, p. H, salinity, etc.
Lipids �Large class of large biomolecules that does not include true polymers �All lipids share one important trait: �They mix poorly, hydrophobic, hydrocarbons Lipids include: 1. Fats 2. Phospholipids 3. Steroids 4. Waxes and pigments
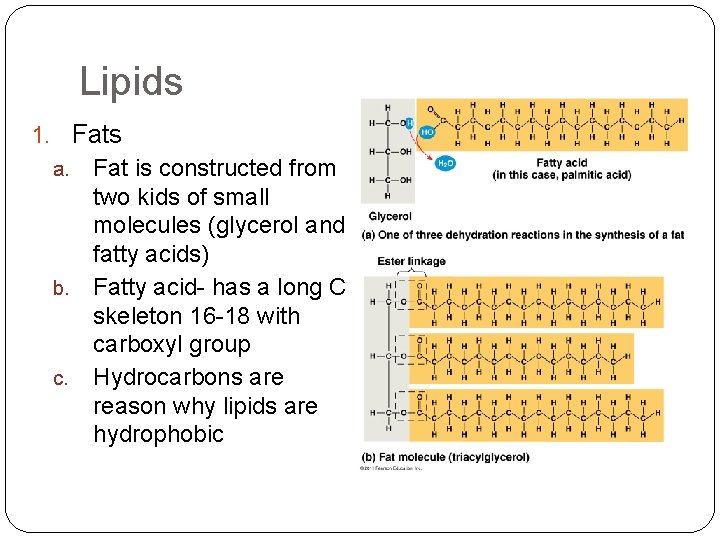
Lipids 1. Fats Fat is constructed from two kids of small molecules (glycerol and fatty acids) b. Fatty acid- has a long C skeleton 16 -18 with carboxyl group c. Hydrocarbons are reason why lipids are hydrophobic a.
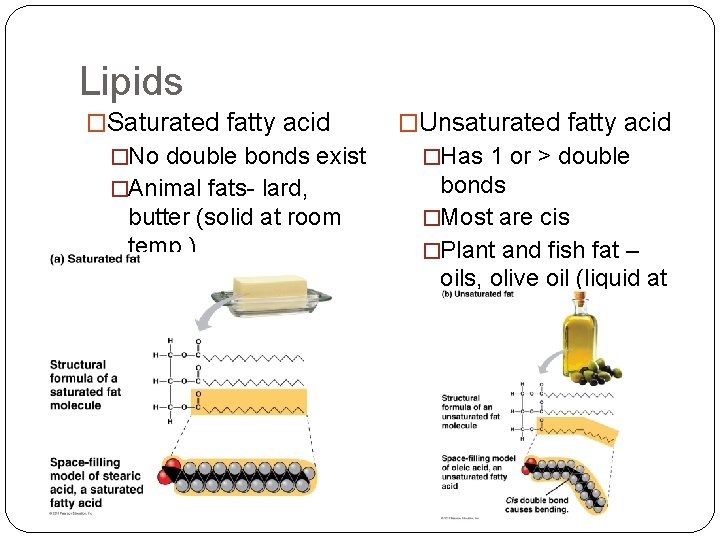
Lipids �Saturated fatty acid �Unsaturated fatty acid �No double bonds exist �Has 1 or > double �Animal fats- lard, bonds �Most are cis �Plant and fish fat – oils, olive oil (liquid at room temp. ) butter (solid at room temp. )
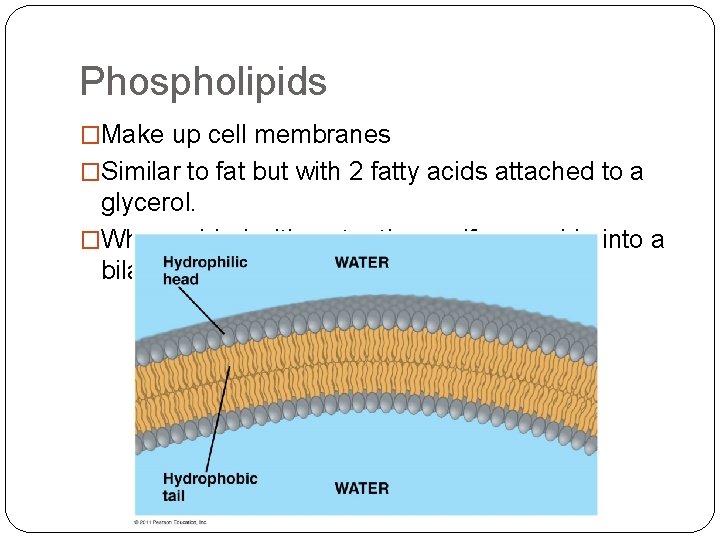
Phospholipids �Make up cell membranes �Similar to fat but with 2 fatty acids attached to a glycerol. �When added with water they self assemble into a bilayer
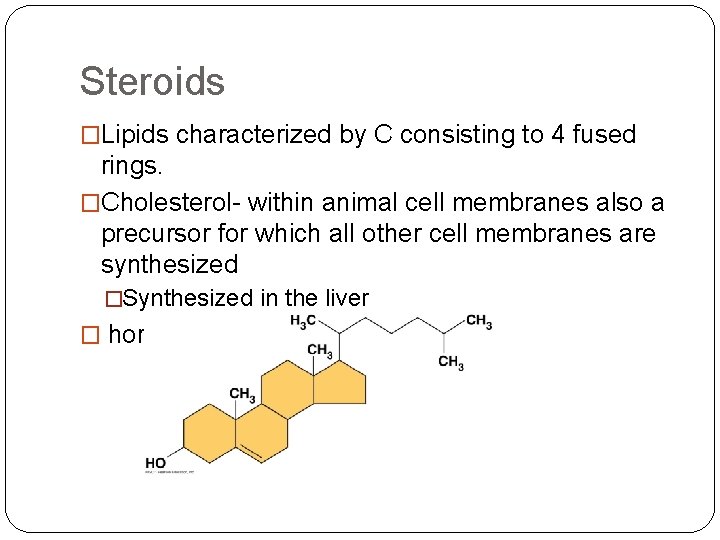
Steroids �Lipids characterized by C consisting to 4 fused rings. �Cholesterol- within animal cell membranes also a precursor for which all other cell membranes are synthesized �Synthesized in the liver � hormones
Nucleic Acids �Genes- units of DNA �Nucleic acids- polymers made of monomers called nucleotides Roles of Nucleic Acids 1. DNA � Genetic material that organisms inherit from their parents 2. RNA � Interacts with cell’s protein synthesizing machinery part of a protein
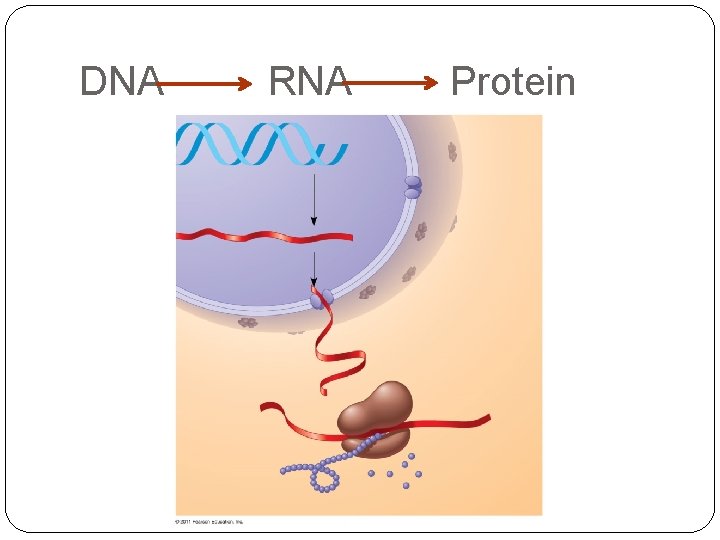
DNA RNA Protein
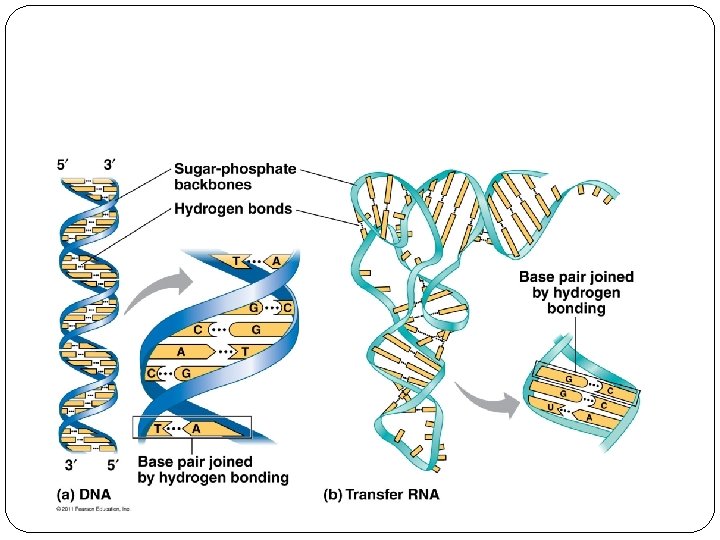
DNA vs RNA DNA RNA �Double stranded �Single stranded �Deoxyribose sugar �Ribose sugar �Thymine �Uracil
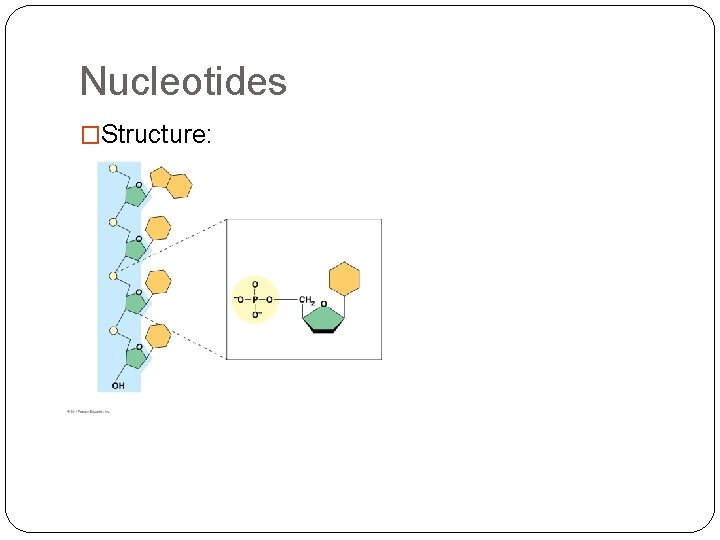
Nucleotides �Structure:
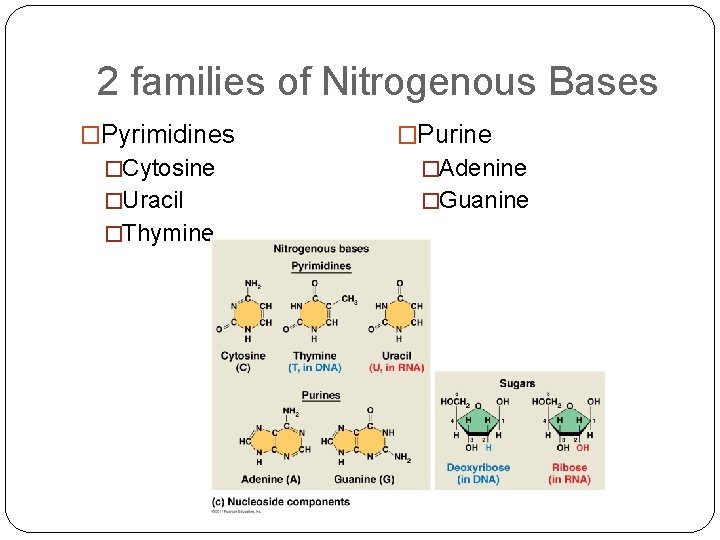
2 families of Nitrogenous Bases �Pyrimidines �Purine �Cytosine �Adenine �Uracil �Guanine �Thymine
- Slides: 36