AOSS 401 Geophysical Fluid Dynamics Atmospheric Dynamics Prepared













![Scale height for isothermal atmosphere Units [R] = J/(kg*K)=kg*m*m/(s*s*kg*K), [T] = K [RT]=m*m/(s*s) [RT/g]=m Scale height for isothermal atmosphere Units [R] = J/(kg*K)=kg*m*m/(s*s*kg*K), [T] = K [RT]=m*m/(s*s) [RT/g]=m](https://slidetodoc.com/presentation_image_h/865a229c83ec272e59f6d408a33e9e36/image-31.jpg)

















- Slides: 70

AOSS 401 Geophysical Fluid Dynamics: Atmospheric Dynamics Prepared: 20131001 Conservation, Energy, Wave Equation Richard B. Rood (Room 2525, SRB) rbrood@umich. edu 734 -647 -3530 Cell: 301 -526 -8572

Class News • Ctools site (AOSS 401 001 F 13) • First Examination on October 22, 2013 • Second Examination on December 10, 2013 • Homework will be posted later today.

Weather • National Weather Service – Model forecasts: • Weather Underground – Model forecasts: • NCAR Research Applications Program

Outline • • Conservation Equation Conservation of Energy Full Equations of Motion Buoyancy / Wave Equation

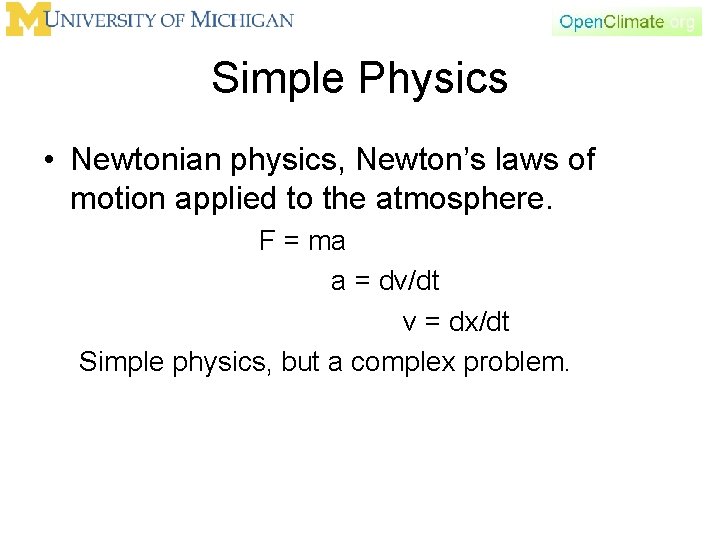
Simple Physics • Newtonian physics, Newton’s laws of motion applied to the atmosphere. F = ma a = dv/dt v = dx/dt Simple physics, but a complex problem.

Conservation (continuity) principle • There are certain parameters, for example, momentum, mass (air, water, ozone, number of atoms, … ), energy, that are conserved. – This is “classical” physics – Simple stuff, like billiard balls hitting each other, ice melting • Conserved? That means that in an isolated system that the parameter remains constant; it’s not created; it’s not destroyed. • Isolated system? A collection of things, described by the parameter, that might interact with each other, but does not interact with other things. That is, nothing new comes into the system – nothing leaves. – If you put a boundary around it then nothing crosses the boundary • Is the Earth an isolated system?

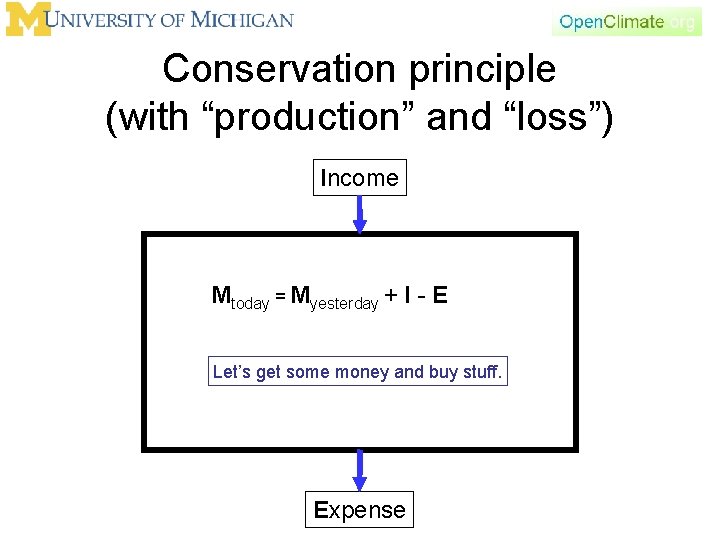
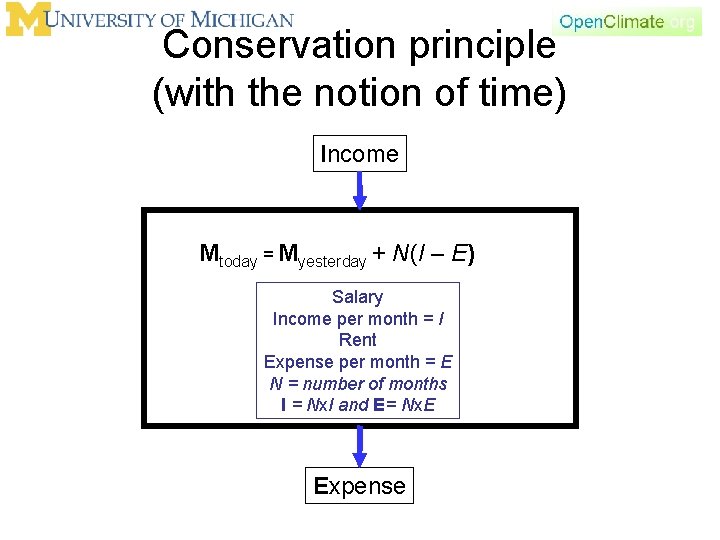
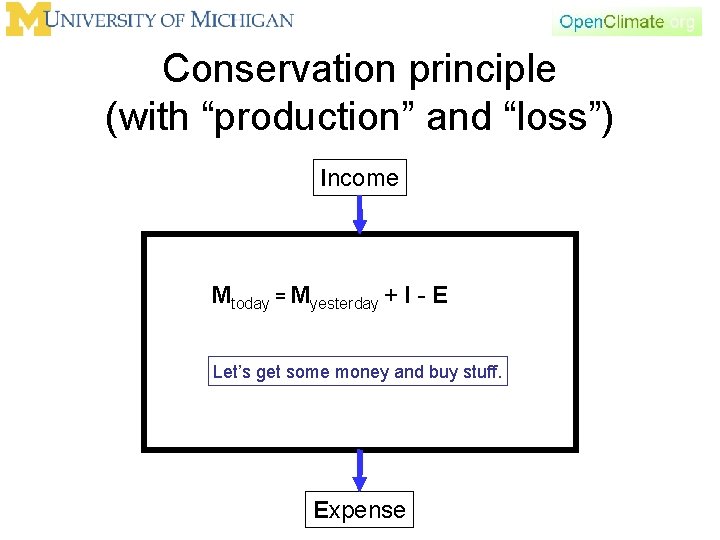
Conservation principle • There are many other things in the world that we can think of as conserved. For example, money. – We have the money that we have. • If we don’t spend money or make money, then the money we have today is the same as the money we had yesterday. Mtoday = Myesterday That’s not very interesting, or realistic

Conservation principle (with “production” and “loss”) Income Mtoday = Myesterday + I - E Let’s get some money and buy stuff. Expense

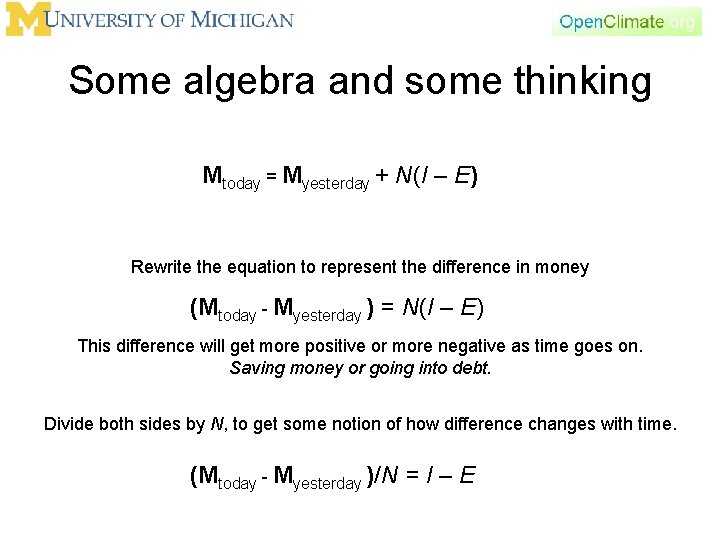
Conservation principle (with the notion of time) Income Mtoday = Myesterday + N(I – E) Salary Income per month = I Rent Expense per month = E N = number of months I = Nx. I and E= Nx. E Expense

Some algebra and some thinking Mtoday = Myesterday + N(I – E) Rewrite the equation to represent the difference in money (Mtoday - Myesterday ) = N(I – E) This difference will get more positive or more negative as time goes on. Saving money or going into debt. Divide both sides by N, to get some notion of how difference changes with time. (Mtoday - Myesterday )/N = I – E

Conservation principle • d. M/dt = Income (per time) – Expense (per time) • What we have done is a dimensional analysis – Start out with money (dollars) – Then we introduced the idea of time • Income per month • Expense per month – Then we divided by time to get some idea of how things change with time

Conservation principle (with “production” and “loss”) Income Mtoday = Myesterday + I - E Let’s get some money and buy stuff. Expense

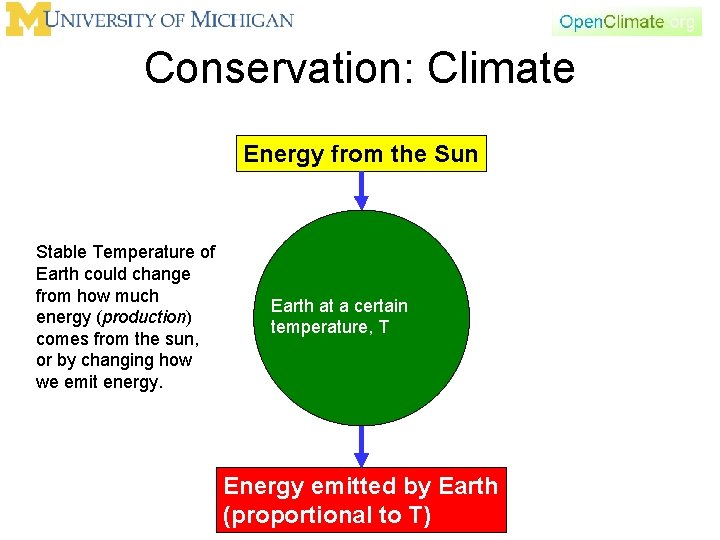
Conservation: Climate Energy from the Sun Stable Temperature of Earth could change from how much energy (production) comes from the sun, or by changing how we emit energy. Earth at a certain temperature, T Energy emitted by Earth (proportional to T)

Let’s come back to our class

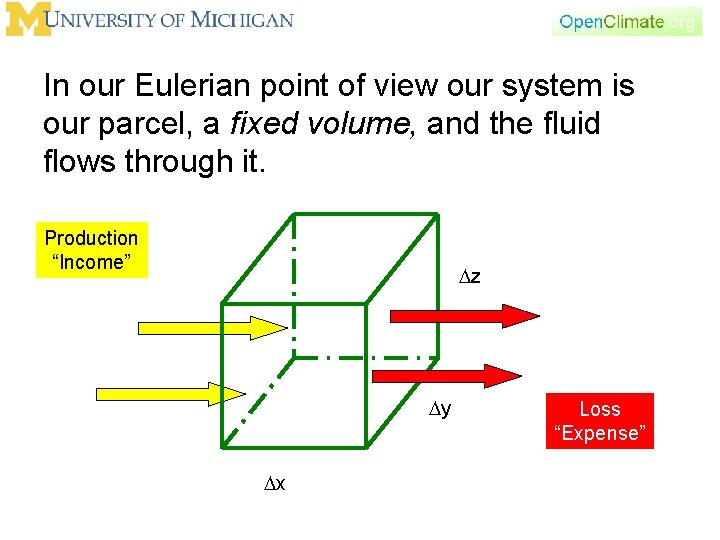
In our Eulerian point of view our system is our parcel, a fixed volume, and the fluid flows through it. Production “Income” Dz Dy Dx Loss “Expense”

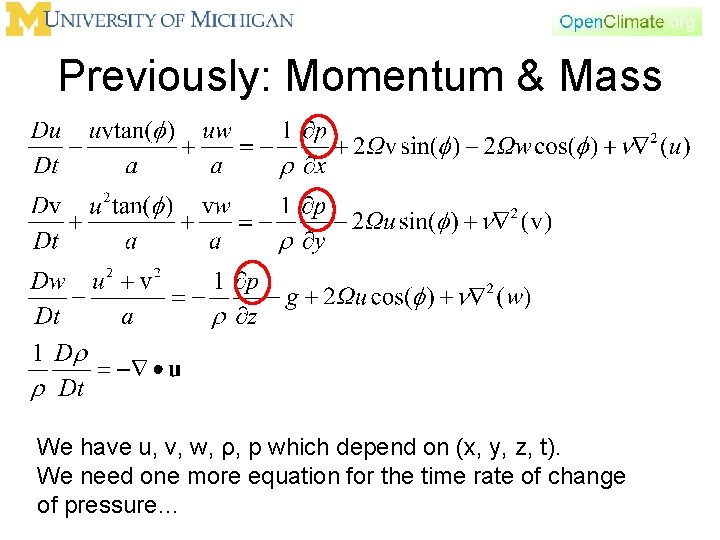
Previously: Momentum & Mass We have u, v, w, ρ, p which depend on (x, y, z, t). We need one more equation for the time rate of change of pressure…

Closing Our System of Equations • We have formed equations to predict changes in motion (conservation of momentum) and density (conservation of mass) • We need one more equation to describe either the time rate of change of pressure or temperature (they are linked through the ideal gas law) • Conservation of energy is the basic principle

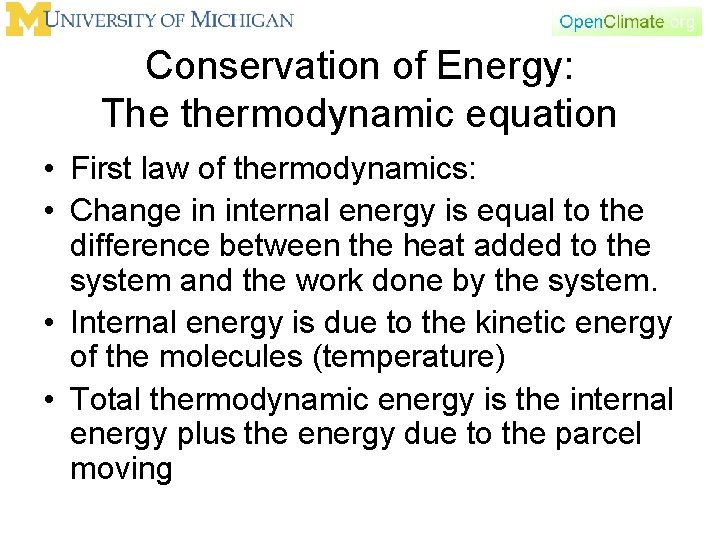
Conservation of Energy: The thermodynamic equation • First law of thermodynamics: • Change in internal energy is equal to the difference between the heat added to the system and the work done by the system. • Internal energy is due to the kinetic energy of the molecules (temperature) • Total thermodynamic energy is the internal energy plus the energy due to the parcel moving

Mathematical Form The mathematical representation follows from the first law of thermodynamics which is stated as: The increase in internal energy of a closed system is equal to the difference of the heat supplied to the system and the work done by it. Many meteorological texts start with the enthalpy form of the first law of thermodynamics, where enthalpy is the total energy of a thermodynamic system. For a closed system this is written as Where h is enthalpy, s is entropy and other variables as previously defined.

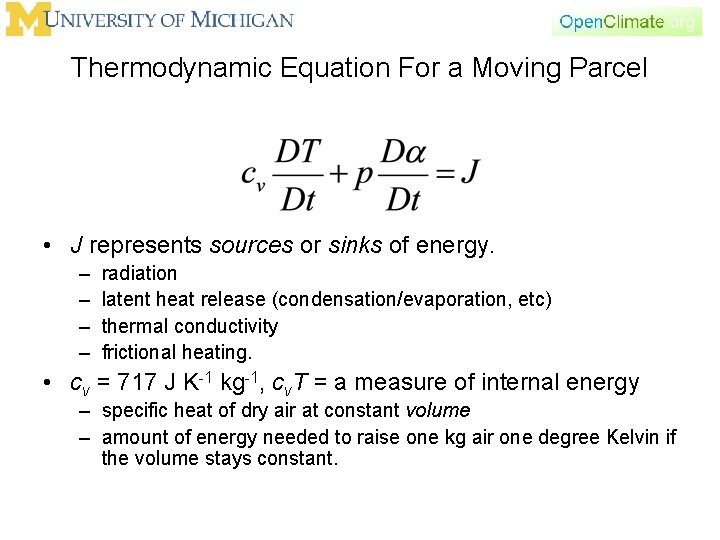
Thermodynamic Equation For a Moving Parcel • J represents sources or sinks of energy. – – radiation latent heat release (condensation/evaporation, etc) thermal conductivity frictional heating. • cv = 717 J K-1 kg-1, cv. T = a measure of internal energy – specific heat of dry air at constant volume – amount of energy needed to raise one kg air one degree Kelvin if the volume stays constant.

Thermodynamic Equation • Involves specific heat at constant volume • Remember the material derivative form of the continuity equation • Following the motion, divergence leads to a change in volume • Reformulate the energy equation in terms of specific heat at constant pressure

Another form of the Thermodynamic Equation • Short derivation • Take the material derivative of the equation of state • Use the product rule and the fact that R=cp-cv • Substitute in from thermodynamic energy equation in Holton • Leads to a prognostic equation for the material change in temperature at constant pressure

(Ideal gas law) (Material derivative) (Chain Rule) (Use R=cp-cv) Substitute in from thermodynamic energy equation (Holton, pp. 47 -49) (Cancel terms)

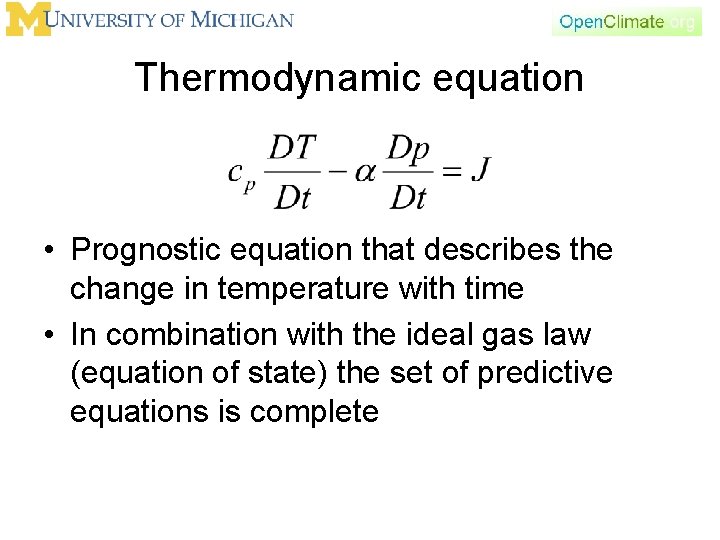
Thermodynamic equation • Prognostic equation that describes the change in temperature with time • In combination with the ideal gas law (equation of state) the set of predictive equations is complete

Atmospheric Predictive Equations Momentum Mass Thermodynamic / Energy Equation of State

Let’s do a problem • Let’s explore the vertical velocity. • Solve for a class of motion.

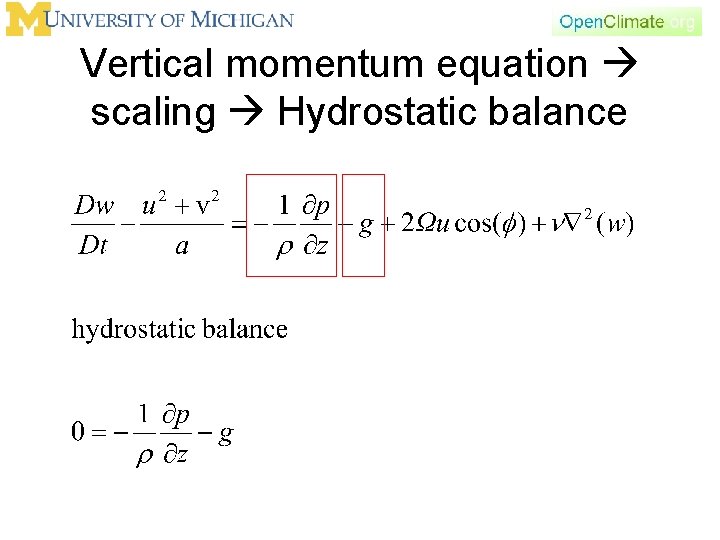
Vertical momentum equation scaling Hydrostatic balance

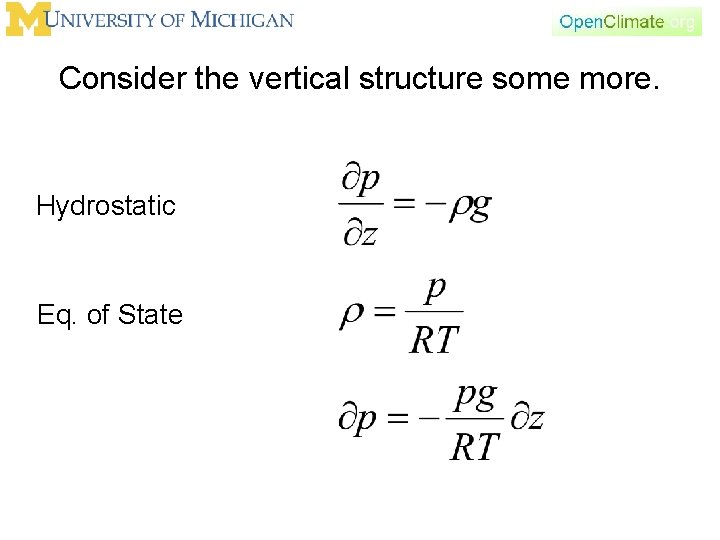
Consider the vertical structure some more. Hydrostatic Eq. of State

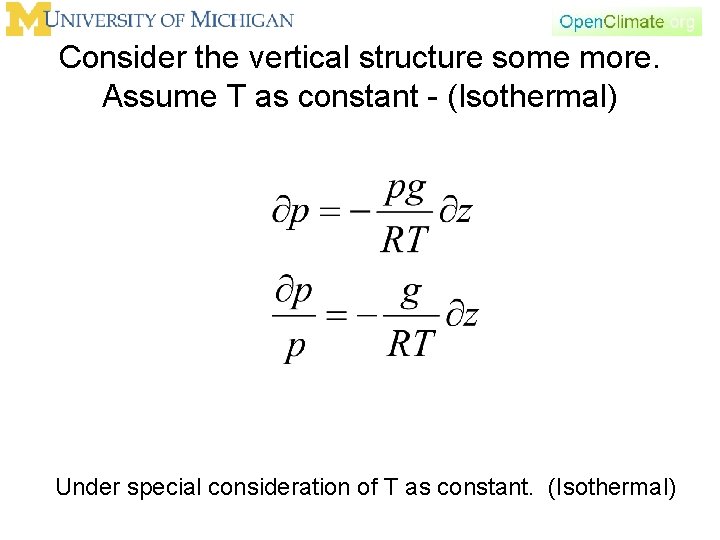
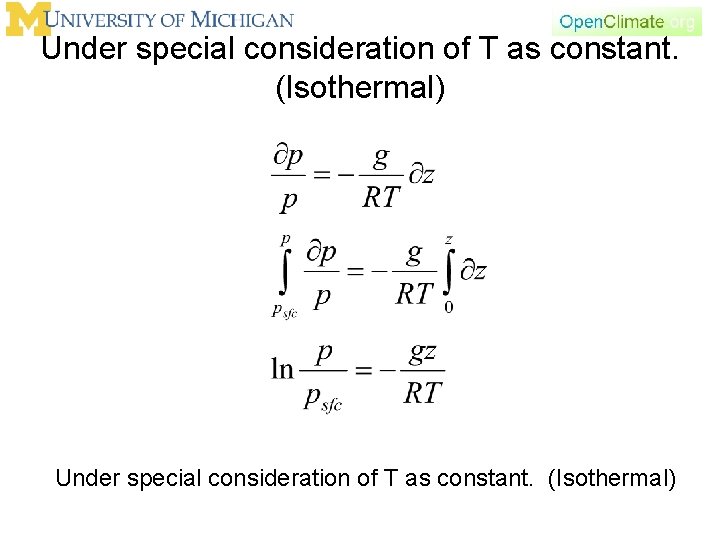
Consider the vertical structure some more. Assume T as constant - (Isothermal) Under special consideration of T as constant. (Isothermal)

Under special consideration of T as constant. (Isothermal)
![Scale height for isothermal atmosphere Units R JkgKkgmmsskgK T K RTmmss RTgm Scale height for isothermal atmosphere Units [R] = J/(kg*K)=kg*m*m/(s*s*kg*K), [T] = K [RT]=m*m/(s*s) [RT/g]=m](https://slidetodoc.com/presentation_image_h/865a229c83ec272e59f6d408a33e9e36/image-31.jpg)
Scale height for isothermal atmosphere Units [R] = J/(kg*K)=kg*m*m/(s*s*kg*K), [T] = K [RT]=m*m/(s*s) [RT/g]=m (unit of length) This gives a number on order of 7 -8 km. Under special consideration of T as constant. (Isothermal)

Pressure altitude Exponential decrease with height

Explore the vertical structure some more • Now let’s consider a non-isothermal atmosphere • What’s the next approximation to temperature that might make sense? – Linear decrease in temperature.

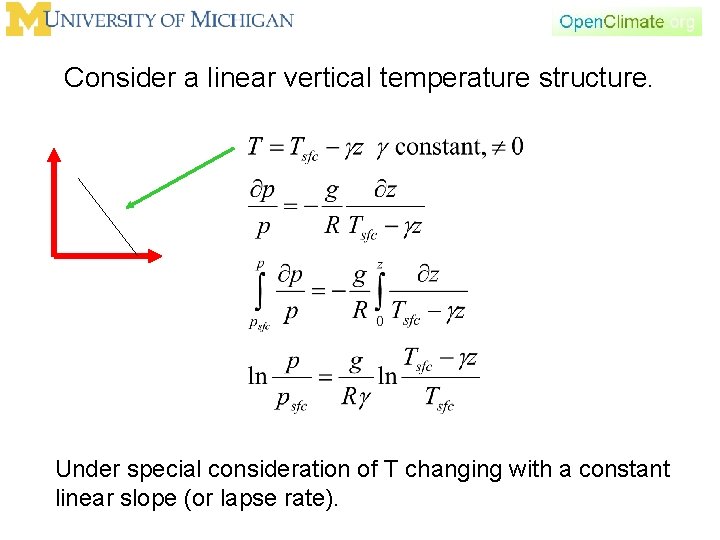
Consider a linear vertical temperature structure. Under special consideration of T changing with a constant linear slope (or lapse rate).

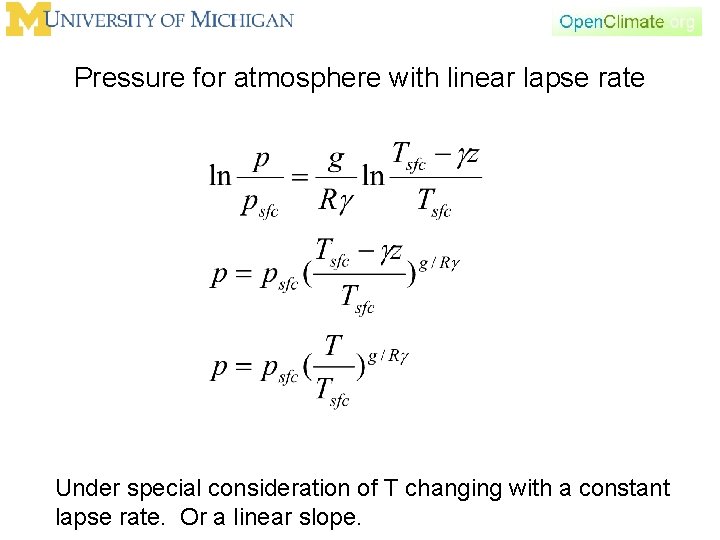
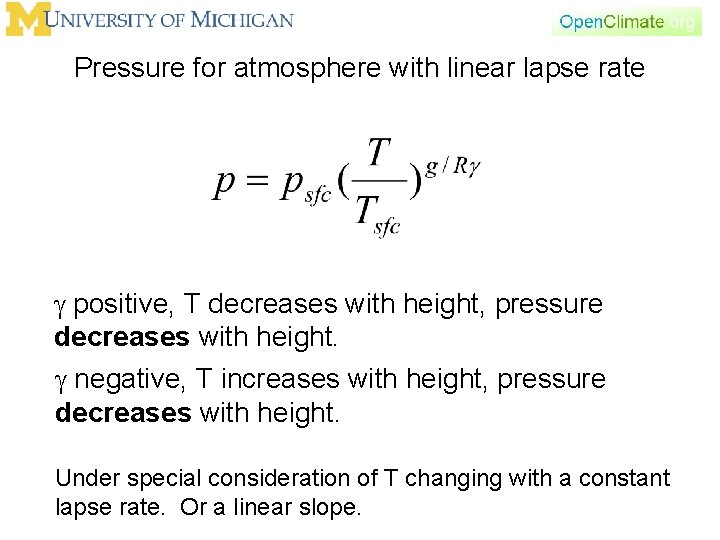
Pressure for atmosphere with linear lapse rate Under special consideration of T changing with a constant lapse rate. Or a linear slope.

Pressure for atmosphere with linear lapse rate g positive, T decreases with height, pressure decreases with height. g negative, T increases with height, pressure decreases with height. Under special consideration of T changing with a constant lapse rate. Or a linear slope.

Consider a parcel in an atmosphere with a linear lapse rate

Let’s return to our linear lapse rate. Under special consideration of T changing with a constant linear slope (or lapse rate).

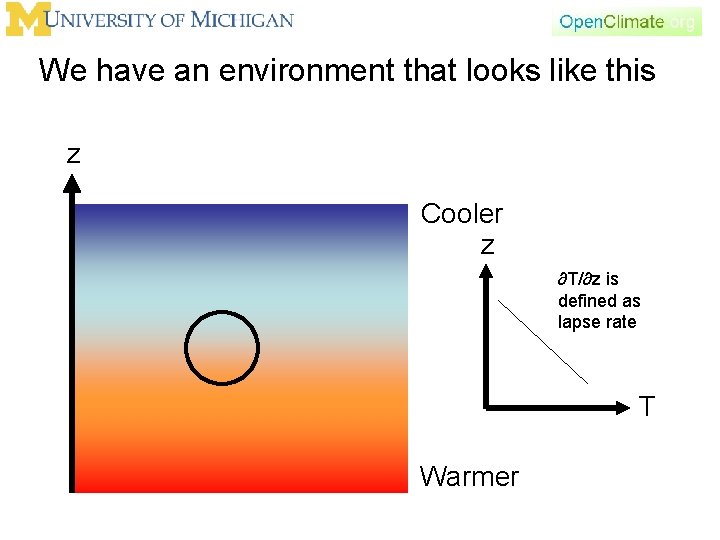
Temperature as function of height z Cooler z ∂T/∂z is defined as lapse rate T Warmer

The parcel method • We are going displace this parcel – move it up and down. – We are going to assume that the pressure adjusts instantaneously; that is, the parcel assumes the pressure of altitude to which it is displaced. – As the parcel is moved its temperature will change according to the adiabatic lapse rate. That is, the motion is without the addition or subtraction of energy. J is zero in thermodynamic equation.

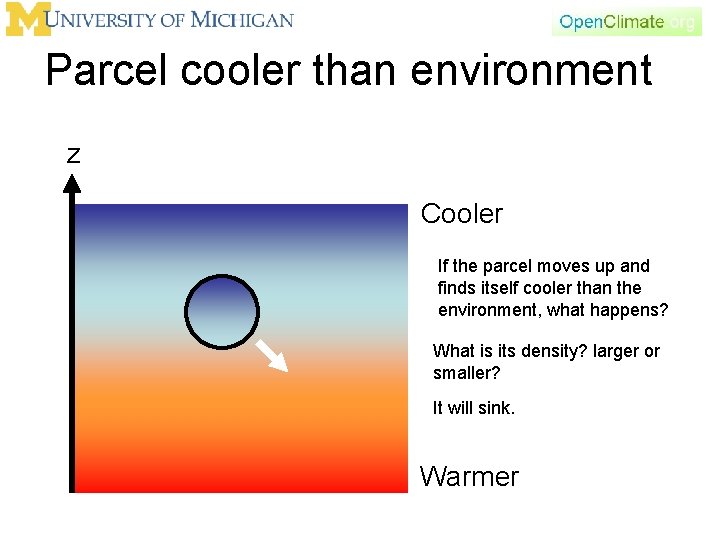
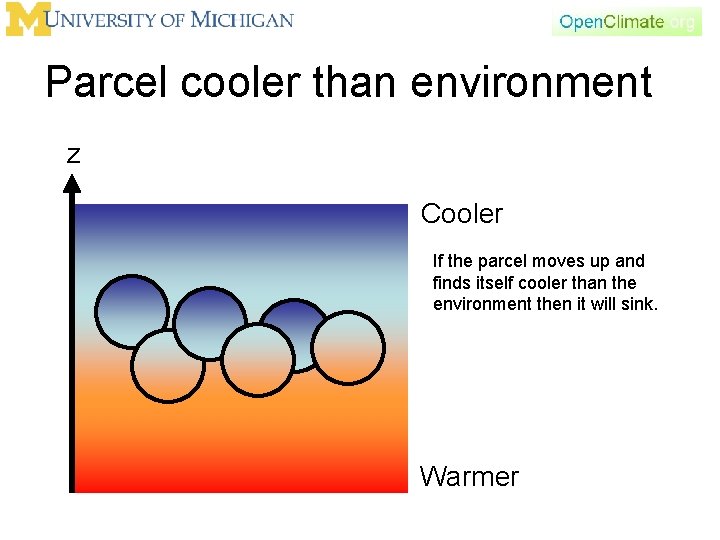
Parcel cooler than environment z Cooler If the parcel moves up and finds itself cooler than the environment, what happens? What is its density? larger or smaller? It will sink. Warmer

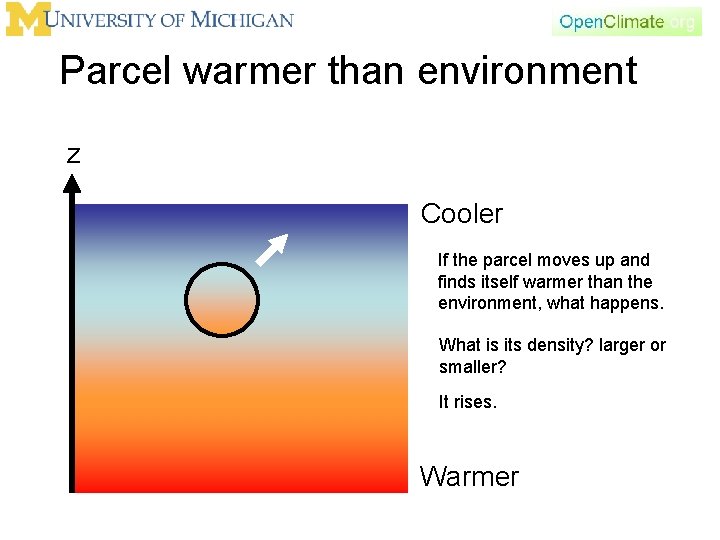
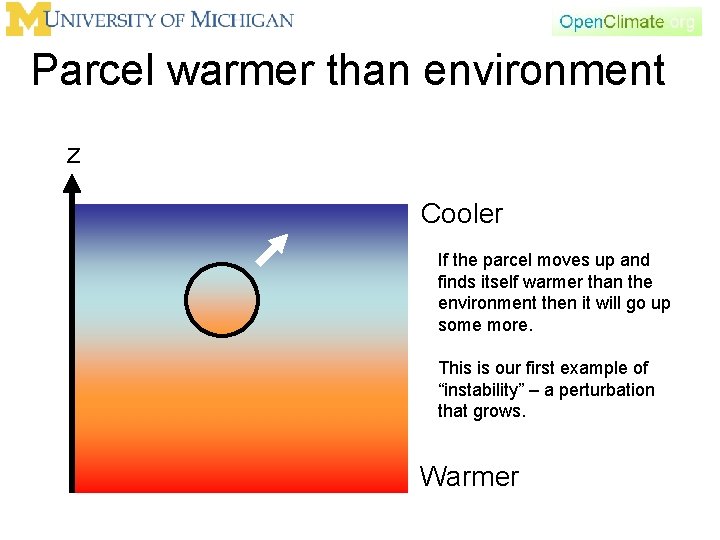
Parcel warmer than environment z Cooler If the parcel moves up and finds itself warmer than the environment, what happens. What is its density? larger or smaller? It rises. Warmer

Parcel warmer than environment z Cooler If the parcel moves up and finds itself warmer than the environment then it will go up some more. This is our first example of “instability” – a perturbation that grows. Warmer

Let’s quantify this. Under consideration of T changing with a constant linear slope (or lapse rate).

Let’s quantify this. Under consideration of T of parcel changing with the dry adiabatic lapse rate

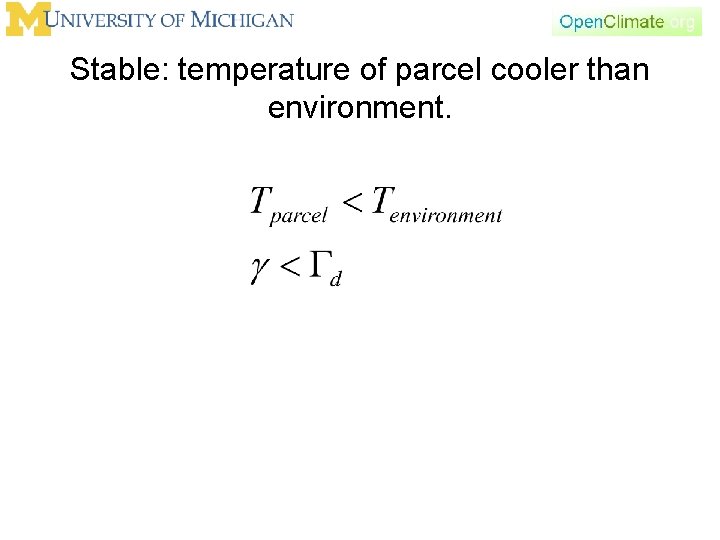
Stable: temperature of parcel cooler than environment.

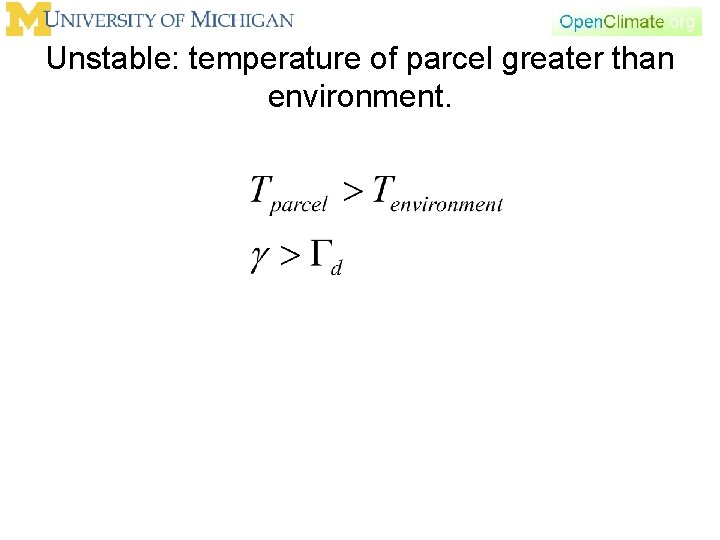
Unstable: temperature of parcel greater than environment.

Stability criteria from physical argument

Let’s return to the vertical momentum equation

We have an environment that looks like this z Cooler z ∂T/∂z is defined as lapse rate T Warmer

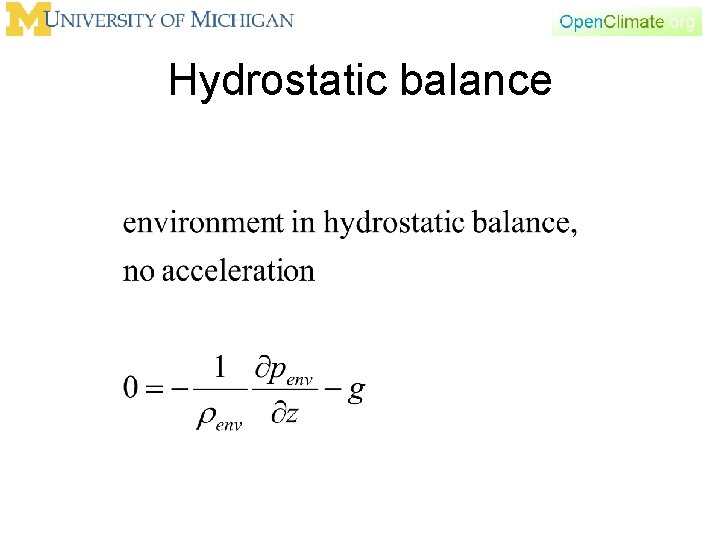
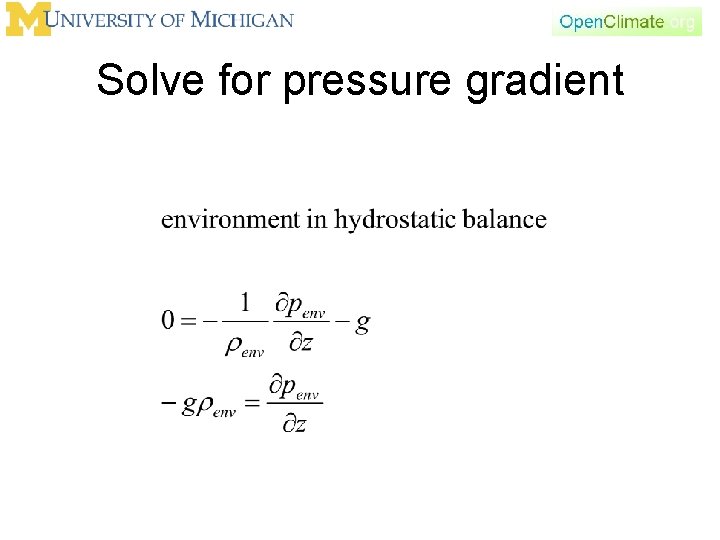
Hydrostatic balance

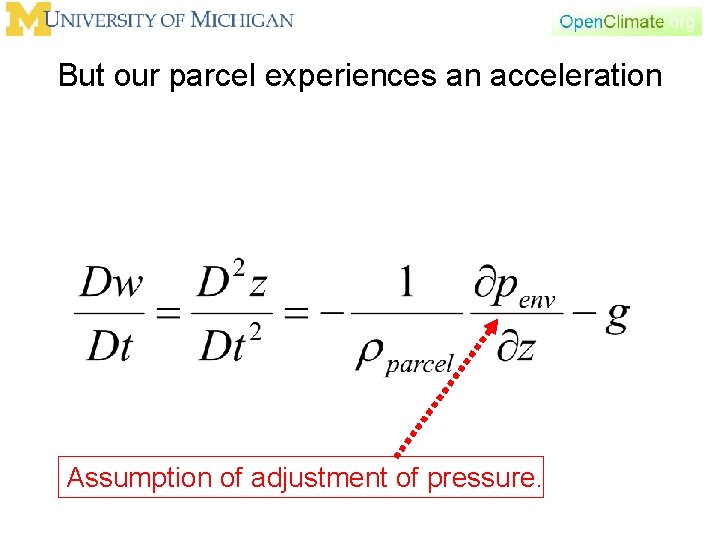
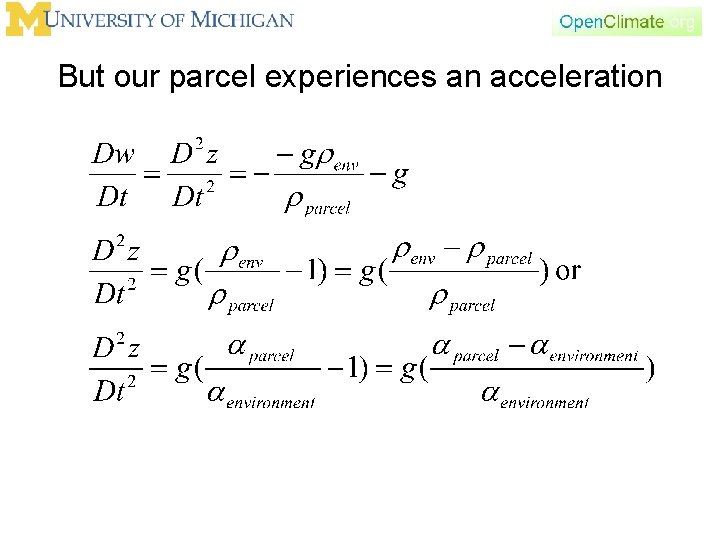
But our parcel experiences an acceleration Assumption of adjustment of pressure.

Solve for pressure gradient

But our parcel experiences an acceleration

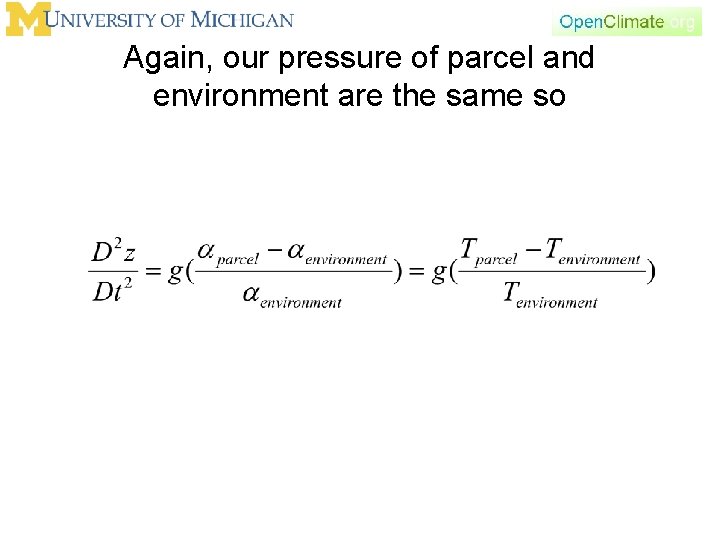
Again, our pressure of parcel and environment are the same so

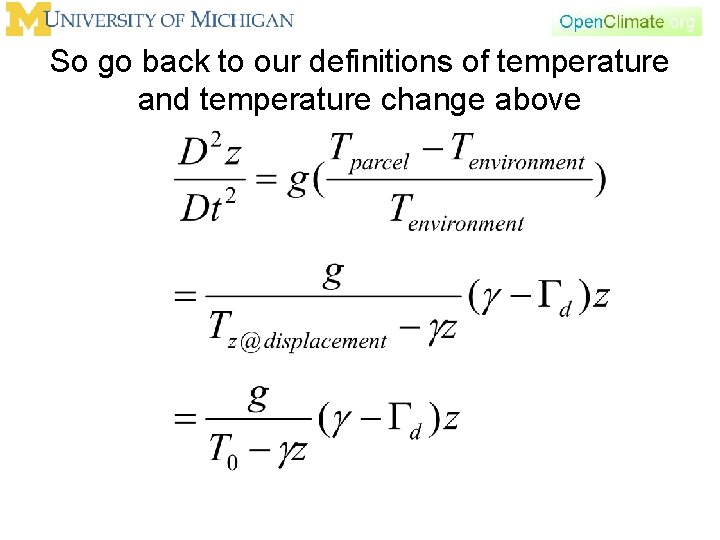
So go back to our definitions of temperature and temperature change above

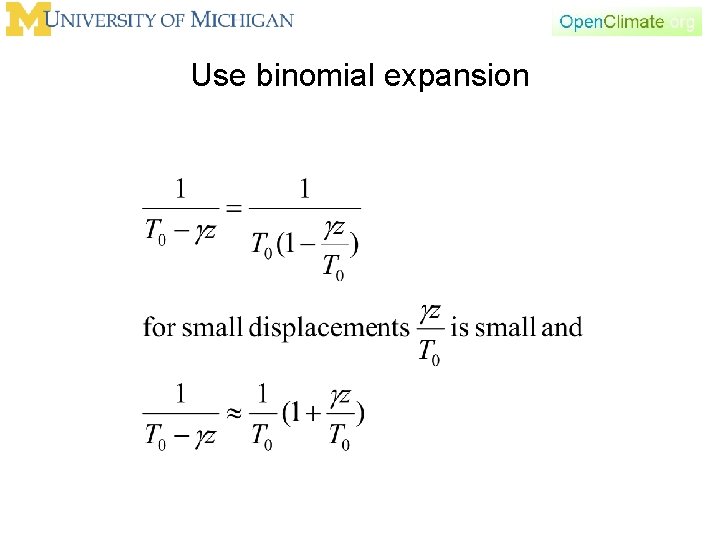
Use binomial expansion

So go back to our definitions of temperature and temperature change above

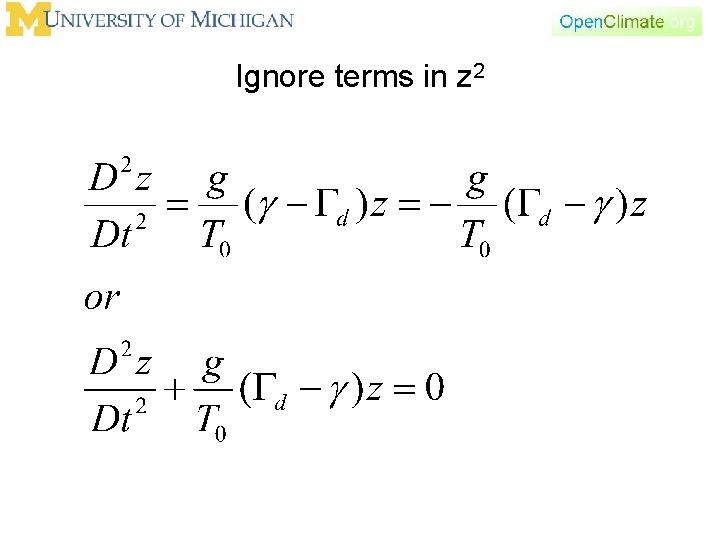
Ignore terms in z 2

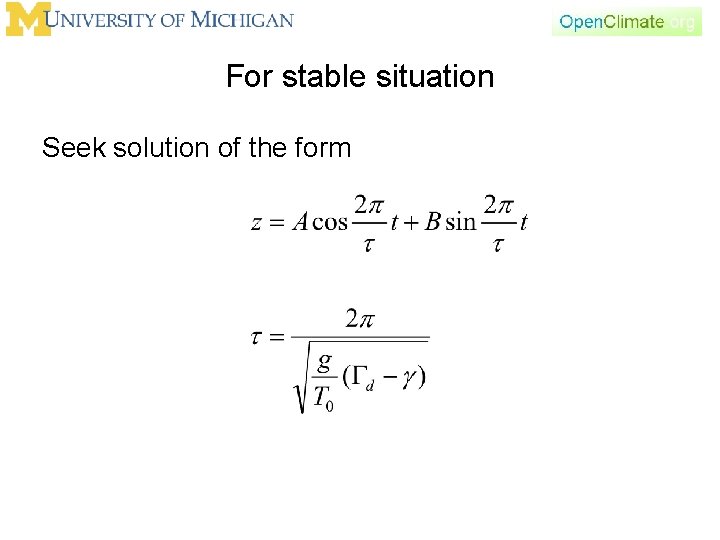
For stable situation Seek solution of the form

For stable situation Seek solution of the form

Parcel cooler than environment z Cooler If the parcel moves up and finds itself cooler than the environment then it will sink. Warmer

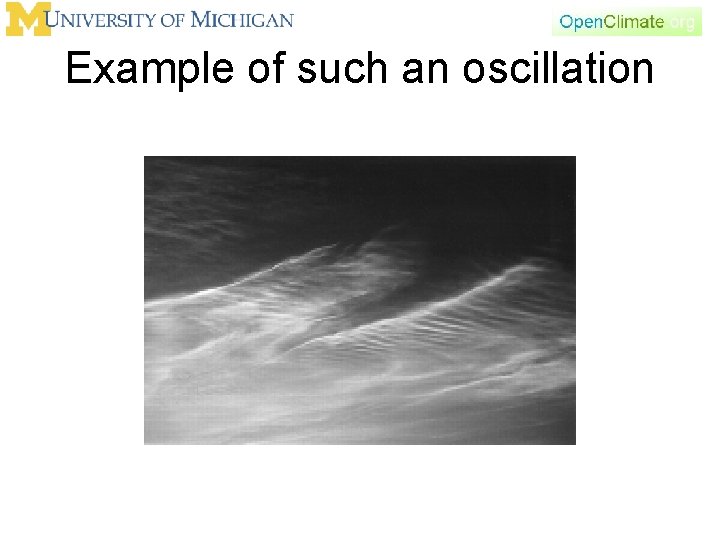
Example of such an oscillation

Waves in my backyard (1)

Waves in my backyard (2)

Waves in my backyard (3)

For unstable situation Seek solution of the form

Parcel warmer than environment z Cooler If the parcel moves up and finds itself warmer than the environment then it will go up some more. This is our first example of “instability” – a perturbation that grows. Warmer

Motion in presence of balance • We scaled the momentum equations and found that vertical accelerations are in general very small. • Then we “allowed” a vertical acceleration in a hydrostatic atmosphere, and found the possibility of vertical motions. – Is this inconsistent in some way?

Skills we have used • • Scale analysis Drawing a picture Decomposed into mean and deviation Assumed functional form of temperature – a model • Series approximation • Wave-like solution – Substitution of assumed form of solution • Multiple perspectives on the same problem