Antimicrobial Stewardship and Microbiology Lab Collaboration in Community

Antimicrobial Stewardship and Microbiology Lab Collaboration in Community Hospitals Continuing Education Webinar January 27, 2021 1

Speakers Ryan K. Shields, Pharm. D, MS Associate Professor of Medicine, University of Pittsburgh Division of Infectious Diseases, UPMC Co-Director, Antibiotic Management Program, UPMC Erin K. Mc. Creary, Pharm. D, BCPS, BCIDP Assistant Professor of Medicine, University of Pittsburgh Division of Infectious Diseases, UPMC Director of Stewardship Innovation, Infectious Disease Connect J. Ryan Bariola, MD, FIDSA Associate Professor of Medicine, University of Pittsburgh Division of Infectious Diseases, UPMC Director, UPMC Outreach Antimicrobial Stewardship Program Director, Antimicrobial Stewardship Telehealth Program, Infectious Disease Connect Kim D’itri, BHS, MT(ASCP) Lead Microbiology Technologist, UPMC Jameson 2

Disclosures • Ryan K. Shields, Pharm. D, MS • Research funding—Accelerate Diagnostics, Allergan, Merck, Melinta, Shionogi, Tetraphase, and Venatorx • Consultant/Advisor/Speaker—Allergan, Menarini, Merck, Pfizer, Shionogi, Nabriva, Entasis, Summit, Venatorx, Utility, Gen. Mark, and T 2 Biosystems • Erin K. Mc. Creary, Pharm. D, BCPS, BCIDP • Advisory Board—Merck, Shionogi, Summit, Entasis, Abb. Vie • J. Ryan Bariola, MD, FIDSA • Research funding — Merck • Kim D’itri, BHS, MT(ASCP) • No disclosures 3

Objectives 1. Describe how antimicrobial stewardship teams and microbiology labs can collaborate to improve antibiotic use 2. Discuss how to track and report data from collaborative projects between microbiology and stewardship teams 3. Determine ways to leverage telemedicine and telehealth to engage antimicrobial stewardship and microbiology labs in community hospitals 4

Dr. Erin Mc. Creary 5

Antimicrobial Stewardship “Coordinated interventions designed to improve and measure the appropriate use of antimicrobials by promoting the selection of the optimal antimicrobial drug regimen, dose, duration of therapy, and route of administration. ” 6

Optimal Stewardship Takes a Village ID-trained MD & Pharm. D Patients and Families Information Technology Prescribing clinicians Pharmacists Team Microbiology Laboratory Administration Infection Prevention Data Analyst Nursing 7

Start with the Basics • Both sides need to • Know what hours/shifts the service is covered • Review existing policies/procedures • Learn current initiatives or goals • Identify barriers • Appreciate current workflows 8

Start with the Basics • Stewardship needs to learn from microbiology: • Antibiogram • AST platform (Vitek, Micro. Scan, etc. ) • How are organisms identified? • CDI testing process (PCR, 2 -step, etc. ) • What rapid diagnostics (if any) are available? • How much workup do you do (if any) on [organism] isolates? AST = Antimicrobial Susceptibility Testing, CDI = Clostridioides difficile infection, PCR = Polymerase Chain Reaction 9

Start with the Basics • Microbiology needs to learn from stewardship: • Patient review process • Recommendation process • Collaboration with consult services (if applicable) • Data tracking/reporting 10
Set Mutual Goals and Work Together to Achieve Them Shadow each other’s workflows Ongoing feedback and data tracking Schedule standing meeting time 11

Breakpoint Updates 12
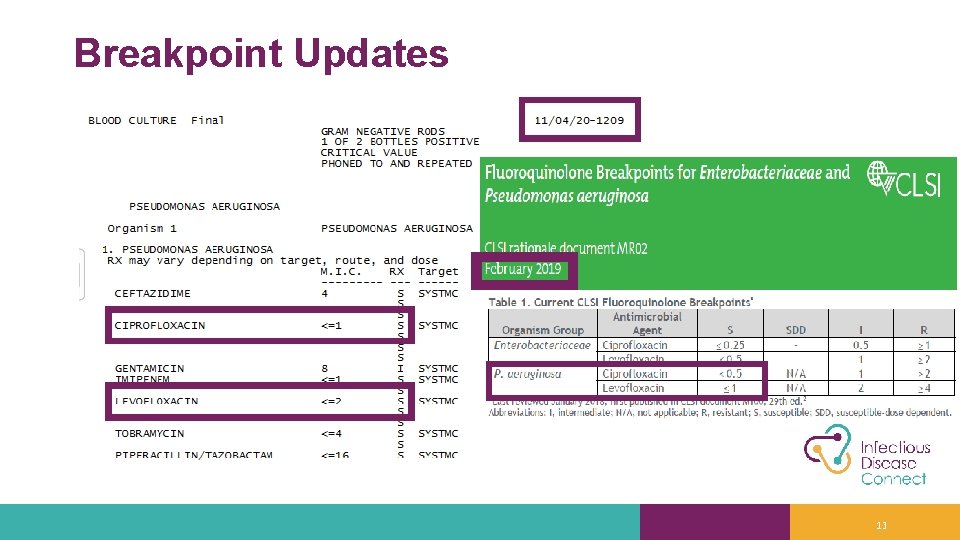
Breakpoint Updates 13

Key Points for Stewardship and Microbiology Collaboration - Breakpoints • Even with stewardship initiatives to decrease FQ use, there are still times they are appropriate drugs and may be needed for patient care • Three options • If panel is FDA-cleared with new breakpoints transition to new breakpoints! • If panel is not FDA-cleared with new breakpoints, but can report low enough MICs lab verification and transition to new breakpoints • If panel is not FDA-cleared and the MICs aren’t reported low enough discuss with stewardship team • Stewardship options • Testing each isolate likely not feasible for the lab • Additional testing upon request requires collaboration • Perhaps only have blood isolates automatically tested? FQ = Fluoroquinolone, FDA = Food and Drug Administration, MIC = Minimum Inhibitory Concentration 14

Breakpoint Updates - Solutions • Prioritize as a team! • Collect data on # of isolates decide if additional validations worthwhile • FQs are useful, but even they may wait • Colistin can be on the back burner • M 100 Free Online • CLSI Webinars • Annual AST update • SIDP/ACCP stewardship updates • AST Rationale Documents • https: //clsi. org/standards/products/packages/mrpkg/ • Annual, free review on Contagion®Live • https: //www. contagionlive. com/view/whats-new-from-the-clsi-subcommittee -on-antimicrobial-susceptibility-testing FQ = Fluoroquinolone, CLSI = Clinical and Laboratory Standards Institute, AST = Antimicrobial Susceptibility Testing SIDP = Society of Infectious Diseases Pharmacists, ACCP = American College of Clinical Pharmacy 15

Breakpoint Updates Solutions • Collaborate when assessing new AST • Can’t have it all • Stewardship and microbiology can determine optimal panels to meet most needs together Panel comparison credit to Ryan K. Shields and Stephanie Mitchell AST = Antimicrobial Susceptibility Testing 16
Other AST Solutions • The return of cefuroxime! • Old panels new panels opportunity • Huge opportunity to ↓ ceftriaxone use! 17

Cascade and/or Suppression Reporting • This can be a good or bad thing • Gram-positives • Usually very helpful • Clindamycin is the first to suppress • Gram-negatives • Easily cuts back on use carbapenems, new BLBLIs • Can make some phenotypic interpretations more difficult • Make backfire, depending how classes are set up • Consider allergies and other patient-specific situations • Consider site of infection – different rules may apply! • Discuss and re-discuss – ensure you’ve thought of almost all scenarios BLBLI = Beta-lactam beta-lactamase inhibitor 18

One of Our Emails: • This happens to all of us! • Triage additional layers of initiatives as they come • Stay focused on goal • Communication and collaboration with all stakeholders essential AS = Antimicrobial Stewardship, MRDO = Multidrug-Resistant Organism 19
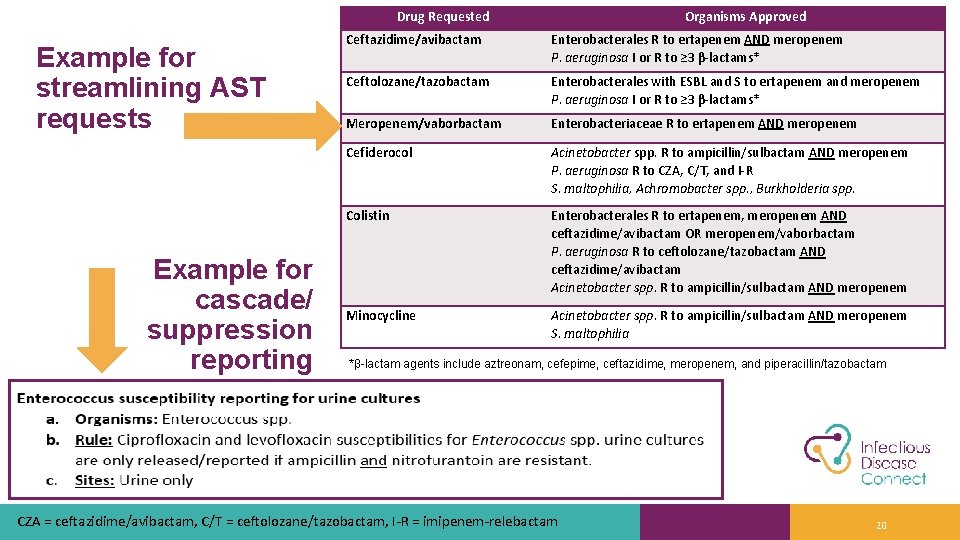
Drug Requested Example for streamlining AST requests Example for cascade/ suppression reporting Organisms Approved Ceftazidime/avibactam Enterobacterales R to ertapenem AND meropenem P. aeruginosa I or R to ≥ 3 β-lactams* Ceftolozane/tazobactam Enterobacterales with ESBL and S to ertapenem and meropenem P. aeruginosa I or R to ≥ 3 β-lactams* Meropenem/vaborbactam Enterobacteriaceae R to ertapenem AND meropenem Cefiderocol Acinetobacter spp. R to ampicillin/sulbactam AND meropenem P. aeruginosa R to CZA, C/T, and I-R S. maltophilia, Achromobacter spp. , Burkholderia spp. Colistin Enterobacterales R to ertapenem, meropenem AND ceftazidime/avibactam OR meropenem/vaborbactam P. aeruginosa R to ceftolozane/tazobactam AND ceftazidime/avibactam Acinetobacter spp. R to ampicillin/sulbactam AND meropenem Minocycline Acinetobacter spp. R to ampicillin/sulbactam AND meropenem S. maltophilia *β-lactam agents include aztreonam, cefepime, ceftazidime, meropenem, and piperacillin/tazobactam CZA = ceftazidime/avibactam, C/T = ceftolozane/tazobactam, I-R = imipenem-relebactam 20
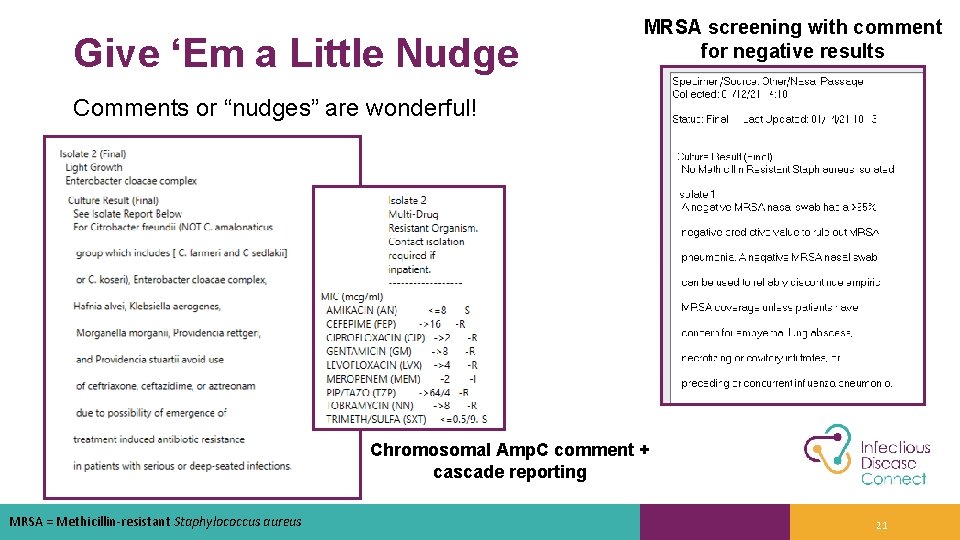
Give ‘Em a Little Nudge MRSA screening with comment for negative results Comments or “nudges” are wonderful! Chromosomal Amp. C comment + cascade reporting MRSA = Methicillin-resistant Staphylococcus aureus 21
A “nudge” works too Musgrove MA, et al. Open Forum Infect Dis 2018; 5(7): ofy 162.
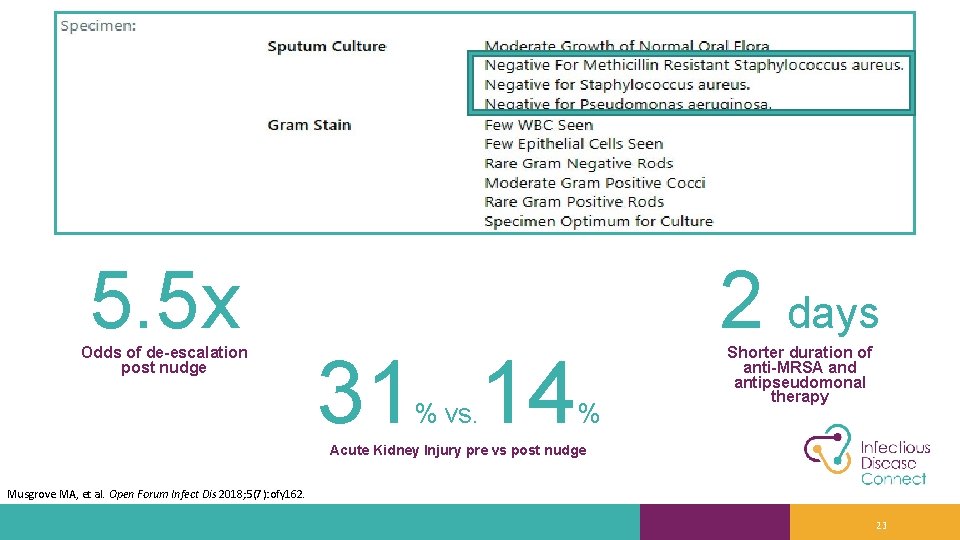
5. 5 x Odds of de-escalation post nudge 31 14 % vs. 2 days % Shorter duration of anti-MRSA and antipseudomonal therapy Acute Kidney Injury pre vs post nudge Musgrove MA, et al. Open Forum Infect Dis 2018; 5(7): ofy 162. 23

Dr. Ryan Bariola 24

• Goal 3: Advance Development and Use of Rapid and Innovative Diagnostic Tests for Identification and Characterization of Resistant Bacteria • 3. 1 Develop and validate new diagnostics—including tests that rapidly distinguish between viral and bacterial pathogens and tests that detect antibiotic-resistance—that can be implemented in a wide range of settings. • 3. 2 Expand the availability and use of diagnostics to improve treatment of antibiotic-resistant bacteria, enhance infection control, and facilitate outbreak detection and response in healthcare and community settings. http: //www. whitehouse. gov/sites/default/files/docs/ national_action_plan_for_combating_antibotic-resistant_bacteria. pdf

Rapid Diagnostics “What goes on in a microbiology laboratory today is very analogous to what Pasteur did in 1850. We take pus and we plate it on seaweed, and then we stick it in an incubator. Then we smell it and Gram stain it and so forth. ” “Where are diagnostics? Well, they're there, but nobody can identify somebody that's willing to pay for the black box. ” “That's what we, as a community, really have to do -- we've got to get on that black box thing. ” -Bartlett JG. Medscape 2006
“Old” Rapid Diagnostics

New Rapid Diagnostics • Expensive • Many to choose from now • Do they matter in smaller hospitals • Expensive • Will providers trust the results • Will providers know when and when not to use them • Will they be outdated in a year • Expensive 28

Limited Evidence of Benefit in “Community Hospitals” Article Community Setting The Impact of Rapid Diagnostic Testing, Surveillance Software, and Clinical Pharmacist Staffing at a Large Community Hospital in the Management of Gram-negative Bloodstream Infections 1 950 bed hospital Integrating Rapid Diagnostics and Antimicrobial Stewardship in Two Community Hospitals Improved Process Measures and Antibiotic Adjustment Time 2 235 beds and 241 beds but part of larger health system with centralized microbiology lab 1. Gawrys GW et al, Diag Microbiol and Infect Dis 2020; 98(1): 115083 2. Lockwood AM, et al. Infect Control Hosp Epidemiol 2016; 37(4): 425 -32 29

Deciding On and Getting What You Need • Micro lab, Stewardship, Pharmacy, and Infection Prevention must work together on evaluations and prioritizations • Make it a need, not a want • Don’t just rely on people like me (physicians) • Societal impact of reduced antimicrobial resistance in the future may be most important yet hardest justification • You don’t need a finance background, but have a solid plan for why it’s needed, how it will be used, and where some cost can be offset • If you’re not a large academic center, find examples from similar hospitals instead • Pro tip: Find out what your competitors are using 30
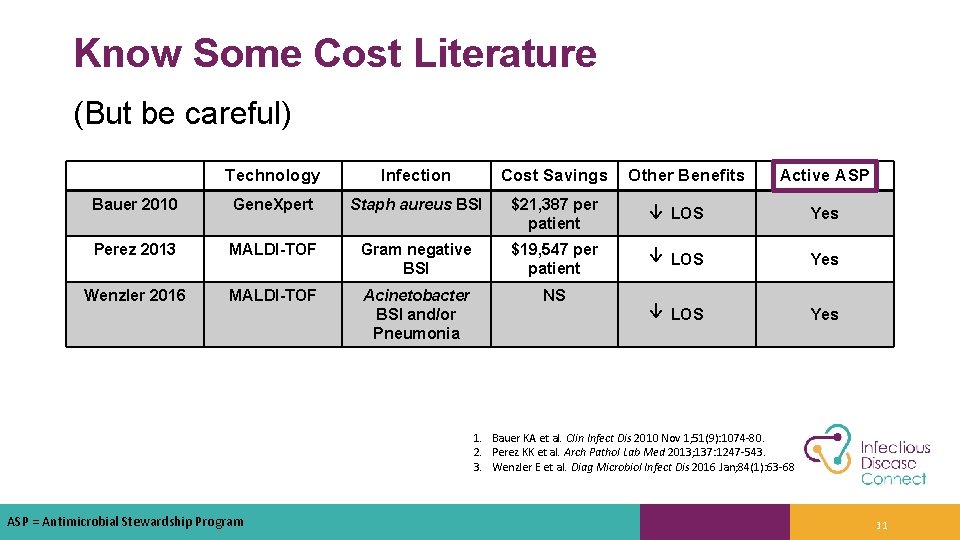
Know Some Cost Literature (But be careful) Technology Infection Cost Savings Other Benefits Active ASP Bauer 2010 Gene. Xpert Staph aureus BSI $21, 387 per patient ↓ LOS Yes Perez 2013 MALDI-TOF Gram negative BSI $19, 547 per patient ↓ LOS Yes Wenzler 2016 MALDI-TOF Acinetobacter BSI and/or Pneumonia NS ↓ LOS Yes 1. Bauer KA et al. Clin Infect Dis 2010 Nov 1; 51(9): 1074 -80. 2. Perez KK et al. Arch Pathol Lab Med 2013; 137: 1247 -543. 3. Wenzler E et al. Diag Microbiol Infect Dis 2016 Jan; 84(1): 63 -68 ASP = Antimicrobial Stewardship Program 31
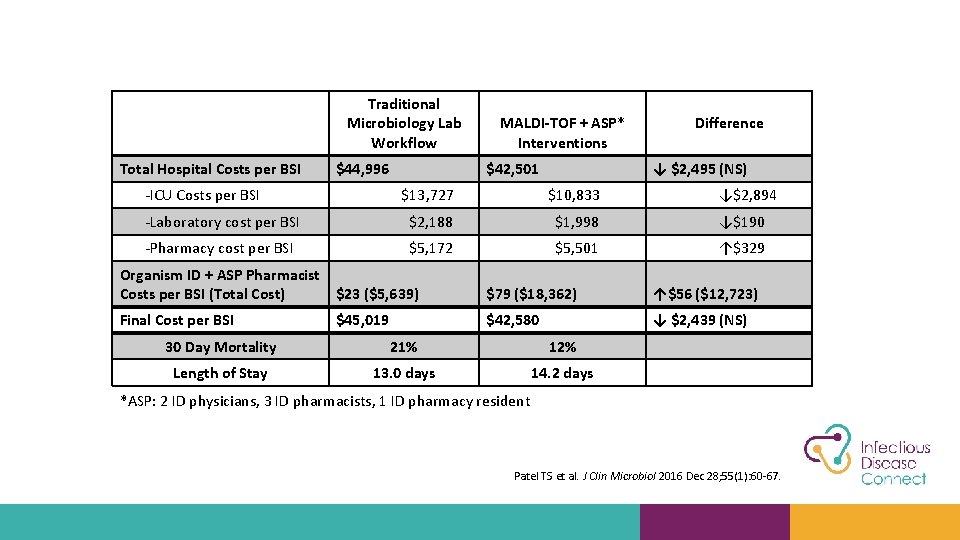
Traditional Microbiology Lab Workflow Total Hospital Costs per BSI $44, 996 -ICU Costs per BSI MALDI-TOF + ASP* Interventions $42, 501 Difference ↓ $2, 495 (NS) $13, 727 $10, 833 ↓$2, 894 -Laboratory cost per BSI $2, 188 $1, 998 ↓$190 -Pharmacy cost per BSI $5, 172 $5, 501 ↑$329 Organism ID + ASP Pharmacist Costs per BSI (Total Cost) $23 ($5, 639) $79 ($18, 362) ↑$56 ($12, 723) Final Cost per BSI $45, 019 $42, 580 ↓ $2, 439 (NS) 30 Day Mortality 21% 12% Length of Stay 13. 0 days 14. 2 days *ASP: 2 ID physicians, 3 ID pharmacists, 1 ID pharmacy resident Patel TS et al. J Clin Microbiol 2016 Dec 28; 55(1): 60 -67.

Moving Beyond Direct Lab Costs • Length of Stay savings • Small improvements don’t really move the financial needle • Ertapenem de-escalation has led to earlier discharges in our experience • Have estimates of cost savings of antibiotic stopping and de-escalation • Reasonable estimates are available for savings from C. diff reductions • Document improvements in micro lab technician efficiency • They are often hard to hire • Have a plan for Diagnostic Stewardship!! • • • Micro and Stewardship need to collaborate on this Doesn’t have to be fancy or 24/7 necessarily Limit unnecessary testing Communication plan for results Ensure providers act on results quickly • Be ready to show results 33

What About Mortality Benefits? 34
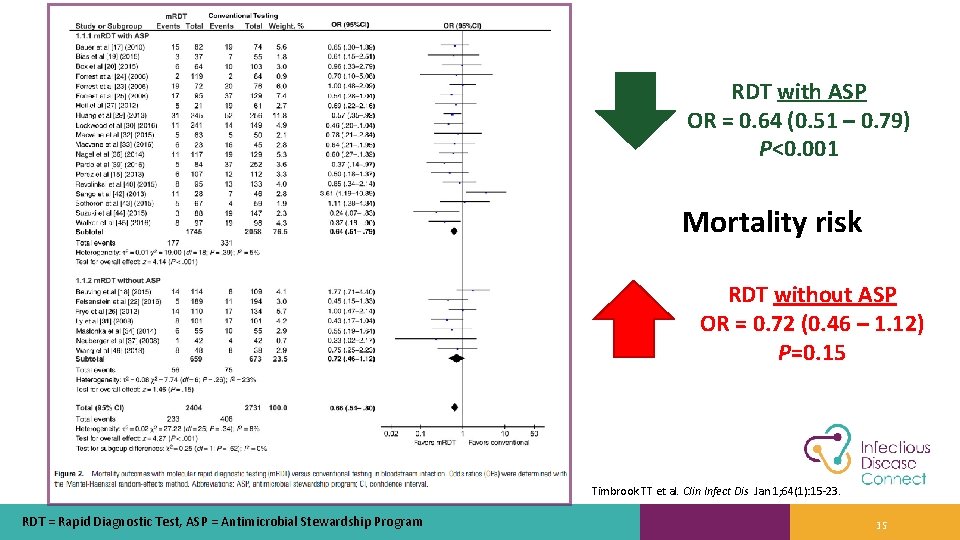
RDT with ASP OR = 0. 64 (0. 51 – 0. 79) P<0. 001 Mortality risk RDT without ASP OR = 0. 72 (0. 46 – 1. 12) P=0. 15 Timbrook TT et al. Clin Infect Dis Jan 1; 64(1): 15 -23. RDT = Rapid Diagnostic Test, ASP = Antimicrobial Stewardship Program 35
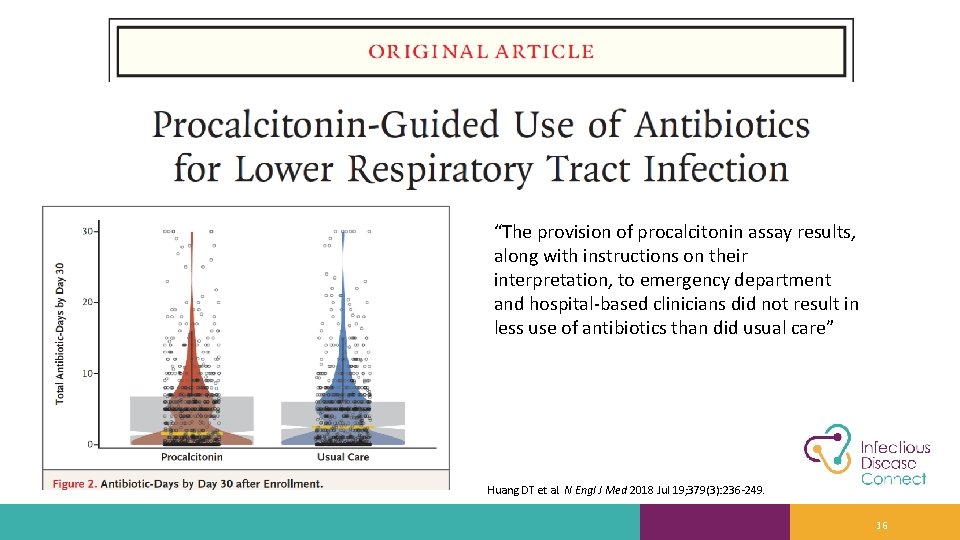
“The provision of procalcitonin assay results, along with instructions on their interpretation, to emergency department and hospital-based clinicians did not result in less use of antibiotics than did usual care” Huang DT et al. N Engl J Med 2018 Jul 19; 379(3): 236 -249. 36

But Wait… • Criticism: • “Implementation in the absence of an antimicrobial stewardship program (ASP) may fail to provide a clinical benefit” 1 • Response: • “We agree with Bremmer et al. that combining two tools – a new diagnostic test and an antimicrobial stewardship program – could be effective and can be tested. We did not design that trial” 2 1. Bremmer DM, Shively NR, Walsh TL N Eng J Med 2018 Nov 15; 379(20): 1972. 2. Huang DT, Yealy DM, Angus DC, the Pro. ACT Investigators N Eng J Med 2018 Nov 15; 379(20): 1973. 37

Staph aureus: Often a good place to start • Multiple assays (genotypic and phenotypic) for ID of Staphylococcus aureus and detection of methicillin resistance from positive blood cultures and other body sites • Is Staphylococcus aureus a problem at your institution? • High rates of Persons Who Inject Drugs? • High rates of CVL’s and Dialysis Catheters? • Some of these platforms allow for expansion to other syndromes or organisms in future • Others aren’t that “fancy” but still can improve turnaround times • • PBB 2 a latex agglutination assay Allows for detection of methicillin resistance from colonies Cheap, doesn’t require upfront expensive equipment Faster than waiting on final susceptibility CVL = Central Venous Line 38
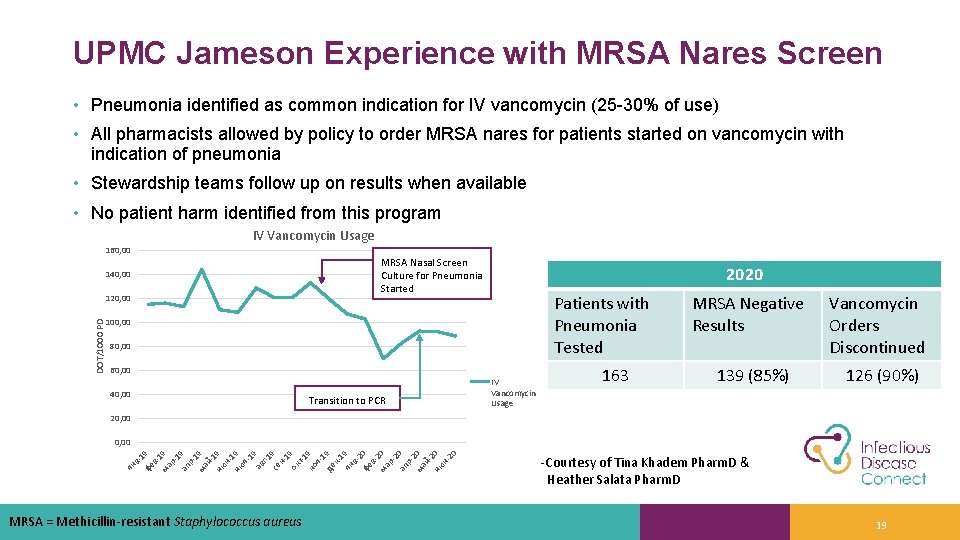
UPMC Jameson Experience with MRSA Nares Screen • Pneumonia identified as common indication for IV vancomycin (25 -30% of use) • All pharmacists allowed by policy to order MRSA nares for patients started on vancomycin with indication of pneumonia • Stewardship teams follow up on results when available • No patient harm identified from this program IV Vancomycin Usage 160, 00 MRSA Nasal Screen Culture for Pneumonia Started 140, 00 DOT/1000 PD 120, 00 2020 Patients with Pneumonia Tested 100, 00 80, 00 60, 00 40, 00 Transition to PCR IV Vancomycin Usage 163 MRSA Negative Results Vancomycin Orders Discontinued 139 (85%) 126 (90%) 20, 00 ян в -1 фе 9 в 1 ма 9 р1 ап 9 р1 ма 9 й 1 ию 9 н 1 ию 9 л 19 ав г-1 се 9 н 1 ок 9 т-1 но 9 я 1 де 9 к 1 ян 9 в 2 фе 0 в 2 ма 0 р2 ап 0 р2 ма 0 й 2 ию 0 н 20 0, 00 MRSA = Methicillin-resistant Staphylococcus aureus -Courtesy of Tina Khadem Pharm. D & Heather Salata Pharm. D 39
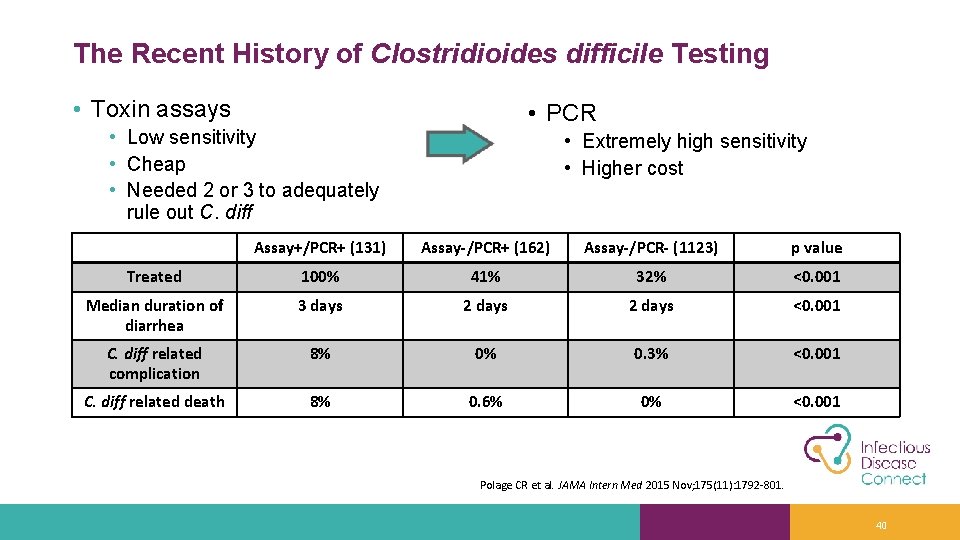
The Recent History of Clostridioides difficile Testing • Toxin assays • PCR • Low sensitivity • Cheap • Needed 2 or 3 to adequately rule out C. diff • Extremely high sensitivity • Higher cost Assay+/PCR+ (131) Assay-/PCR+ (162) Assay-/PCR- (1123) p value Treated 100% 41% 32% <0. 001 Median duration of diarrhea 3 days 2 days <0. 001 C. diff related complication 8% 0% 0. 3% <0. 001 C. diff related death 8% 0. 6% 0% <0. 001 Polage CR et al. JAMA Intern Med 2015 Nov; 175(11): 1792 -801. 40
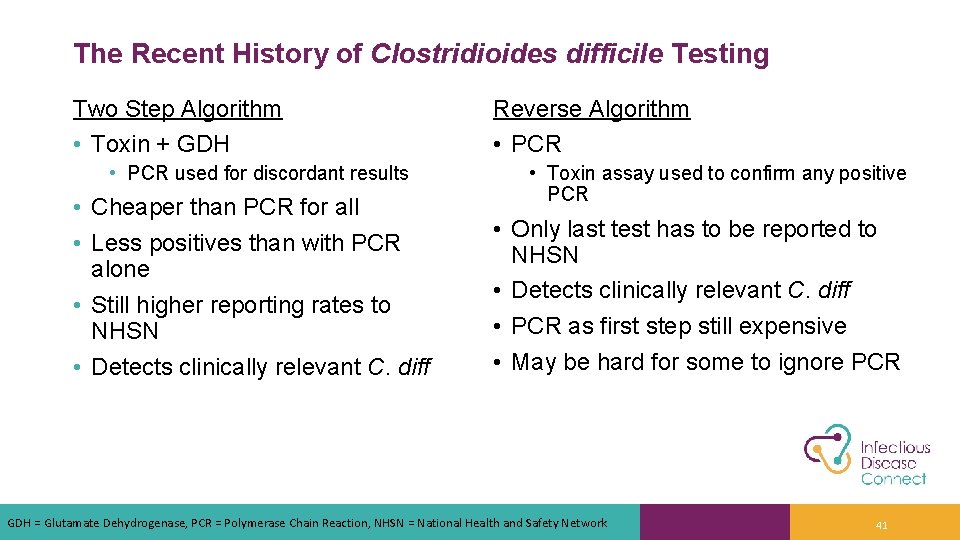
The Recent History of Clostridioides difficile Testing Two Step Algorithm • Toxin + GDH • PCR used for discordant results • Cheaper than PCR for all • Less positives than with PCR alone • Still higher reporting rates to NHSN • Detects clinically relevant C. diff Reverse Algorithm • PCR • Toxin assay used to confirm any positive PCR • Only last test has to be reported to NHSN • Detects clinically relevant C. diff • PCR as first step still expensive • May be hard for some to ignore PCR GDH = Glutamate Dehydrogenase, PCR = Polymerase Chain Reaction, NHSN = National Health and Safety Network 41
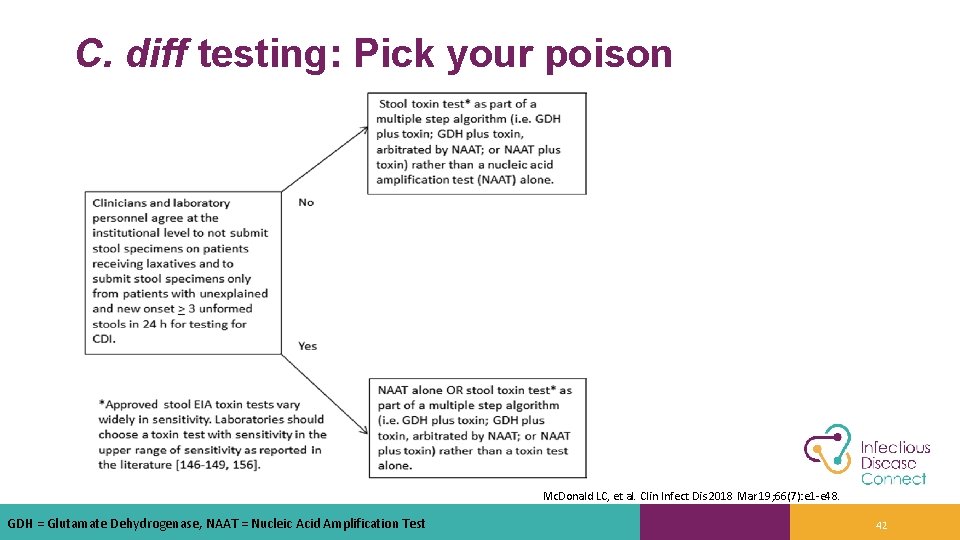
C. diff testing: Pick your poison Mc. Donald LC, et al. Clin Infect Dis 2018 Mar 19; 66(7): e 1 -e 48. GDH = Glutamate Dehydrogenase, NAAT = Nucleic Acid Amplification Test 42
Kim D’itri 43

• 146 bed general hospital (1 hour outside of Pittsburgh, PA) • Jameson Memorial Hospital until joining UPMC Health System in 2018 • 6 months after joining UPMC, became regional micro lab for 3 additional UPMC hospitals (436 beds total) • 116 beds at 2 campuses each 30 -45 minutes away • 174 bed hospital 2 hours away 44

Small Hospital Problems • Minimal involvement with clinicians or pharmacists • Very isolated • “Forgotten department” • After joining a system • Access to more stewardship resources/collaboration • Access to microbiology/pathology experts when needed • Use of UPMC resources for many reference labs now • After becoming a regional lab • • Lots of attention from lab leadership Staffing and limited physical space become issues Managing courier times is important Adjusting workflows around courier schedules 45

Ongoing Challenges and Opportunities • Courier needs reduce the time our lab has to complete workups in a clinically useful time period • Clinicians like having more testing capabilities, but still don’t like delays in turn around times • Regionalization does make justifications for rapid diagnostics easier • Even for potential large capital projects like MALDI-TOF • How much testing to keep local versus send to our regional hub now? • Should the rapid diagnostic tests be centralized or distributed? • Many can be run by non-micro trained staff now 46

Collaborating with Stewardship • • We now participate with Stewardship meetings More people seem to call/visit us now We have clinical resources available when questions arise Recent successes due to collaboration with stewardship: • • • Updated breakpoints for Enterobacterales and Pseudomonas Updated fluoroquinolone breakpoints MRSA PCR implemented CRE testing to be implemented Clinical assistance with making antibiograms relevant and appropriate Ready resource to assist with evaluating clinical need for new equipment and creating justifications CRE = Carbapenem-Resistant Enterobacteriaceae, MRSA = Methicillin-Resistant Staphylococcus aureus 47

Advice • Any micro lab: • Find your stewardship teams and stay involved. Can keep you apprised of needed changes that could help patient care • Keep them updated on new changes in micro lab (new CAP questions on stewardship for example) • Involve stewardship in any evaluation of rapid diagnostics • Make sure you have a plan with stewardship on how new tests will be used and how they will be monitored • Regional micro lab: • • Don’t sacrifice too much turn around time if possible Decide what really needs to be sent to regional lab Have a frequent and reliable courier schedule Leverage increased workload for new equipment that may be appropriate CAP = Community-Acquired Pneumonia 48

CE CODE: QUVSOM Step 1: Make sure you create an Ethos account at http: //cce. upmc. com Step 2: Enter code at http: //cce. upmc. com/code Step 3: Go to My Account, My Courses, Choose Pending Activities Step 4: Click on the course title to complete the course evaluation 49

Questions? Ryan K. Shields, Pharm. D, MS Associate Professor of Medicine, University of Pittsburgh Division of Infectious Diseases, UPMC Co-Director, Antibiotic Management Program, UPMC Erin K. Mc. Creary, Pharm. D, BCPS, BCIDP Assistant Professor of Medicine, University of Pittsburgh Division of Infectious Diseases, UPMC Director of Stewardship Innovation, Infectious Disease Connect J. Ryan Bariola, MD, FIDSA Associate Professor of Medicine, University of Pittsburgh Division of Infectious Diseases, UPMC Director, UPMC Outreach Antimicrobial Stewardship Program Director, Antimicrobial Stewardship Telehealth Program, Infectious Disease Connect Kim D’itri, BHS, MT(ASCP) Lead Microbiology Technologist, UPMC Jameson 50

Thank you! 51

Post-Test Question 1 • Which of the following is an important area where microbiology labs and stewardship teams can collaborate? A. Breakpoint updates B. Antibiotic purchasing C. Automatic IV to PO policy D. Renal dosing chart modifications 52

Post-Test Question 2 • What is a free, online resource microbiology laboratories and stewardship teams can access for breakpoint changes and susceptibility testing guidance of antibiotics? A. CLSI M 28 B. CLSI M 40 C. CLSI M 60 D. CLSI M 100 53

Post-Test Question 3 • Which of the following rapid diagnostics tests described in this presentation helped a community hospital stewardship program decrease vancomycin days of therapy? A. Procalcitonin B. MRSA nasal screening via PCR C. Respiratory sample “rule out” comment D. Nucleic acid amplification test 54

Additional Reading Resources • CLSI M 100: CLSI e. Clipse Ultimate Access - Powered by Edaptive Technologies (edaptivedocs. net) • Kuper KM, et al. Antimicrobial susceptibility testing: A primer for clinicians. Pharmacotherapy 2009; 29(11): 1326– 1343. • Barlam TF, et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016; 62(10): e 51– e 77. 55
- Slides: 55