Anticoagulant Review Morning Report April 25 2018 Sydney













- Slides: 29

Anticoagulant Review Morning Report – April 25, 2018 Sydney N. Kutter, Pharm. D. PGY 1 Pharmacy Practice Resident Central Texas Veterans Health Care System

Anticoagulants Warfarin (Coumadin) Edoxaban (Savaysa) Rivaroxaban (Xarelto) Dabigatran (Pradaxa) Apixaban (Eliquis) 2

WARFARIN REVIEW 3

Warfarin - Dosing Highly individualized – tailored based on bleeding risk, indication, goal range, interactions, etc. Initiation between 2. 5 -10 mg daily Lower doses for elderly age, genetic polymorphism, alcohol or tobacco usage, change in diet, interacting medications, sickness, etc. Adjusted based on INR result 4

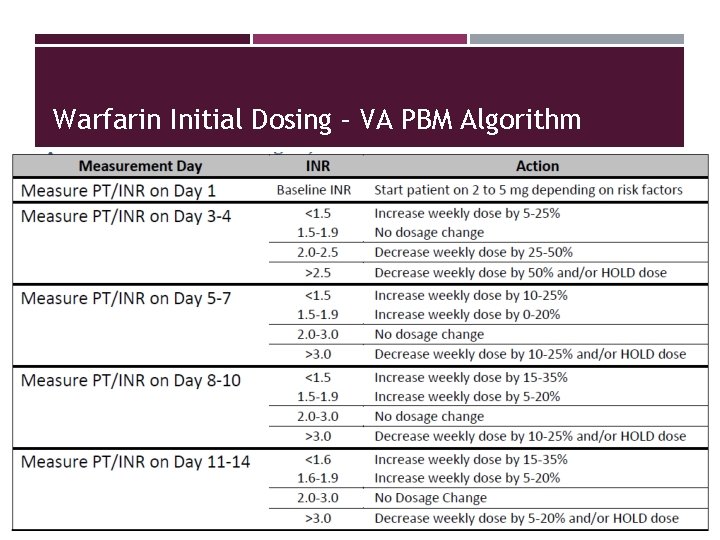
Warfarin Initial Dosing – VA PBM Algorithm

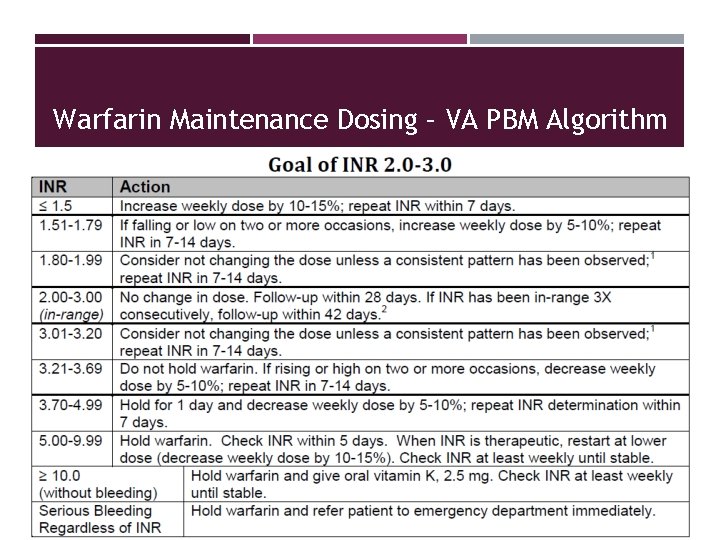
Warfarin Maintenance Dosing – VA PBM Algorithm

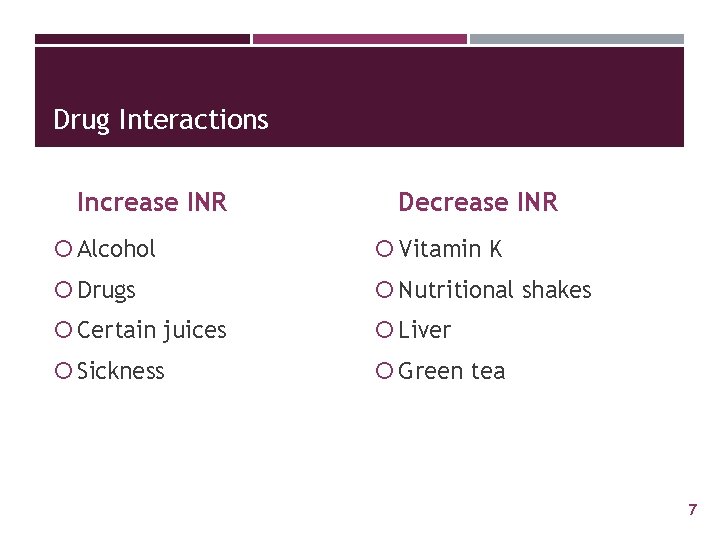
Drug Interactions Increase INR Decrease INR Alcohol Vitamin K Drugs Nutritional shakes Certain juices Liver Sickness Green tea 7

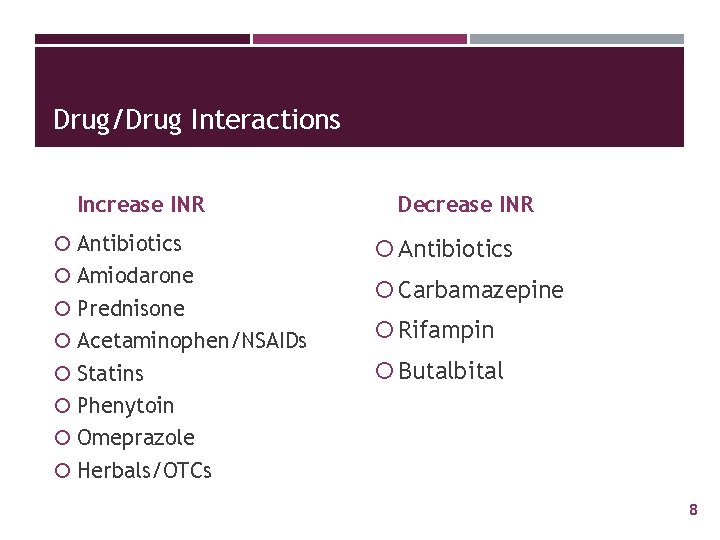
Drug/Drug Interactions Increase INR Antibiotics Amiodarone Prednisone Decrease INR Antibiotics Carbamazepine Acetaminophen/NSAIDs Rifampin Statins Butalbital Phenytoin Omeprazole Herbals/OTCs 8

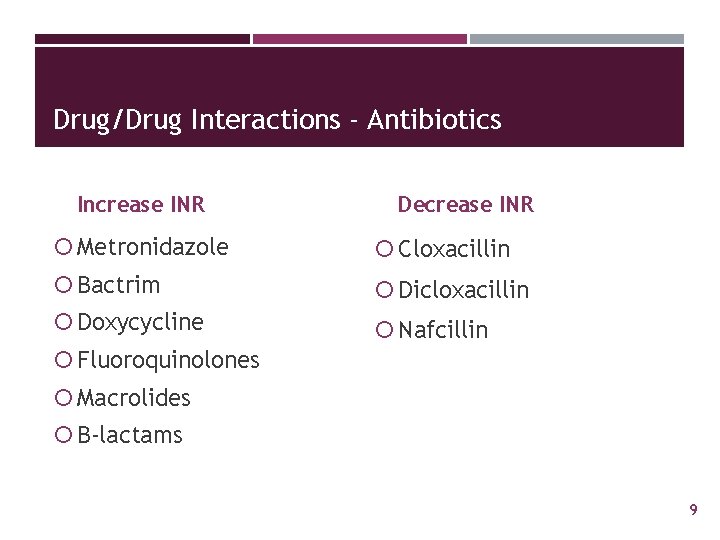
Drug/Drug Interactions - Antibiotics Increase INR Decrease INR Metronidazole Cloxacillin Bactrim Dicloxacillin Doxycycline Nafcillin Fluoroquinolones Macrolides B-lactams 9

DOAC REVIEW DIRECT ORAL ANTICOAGULANTS 10

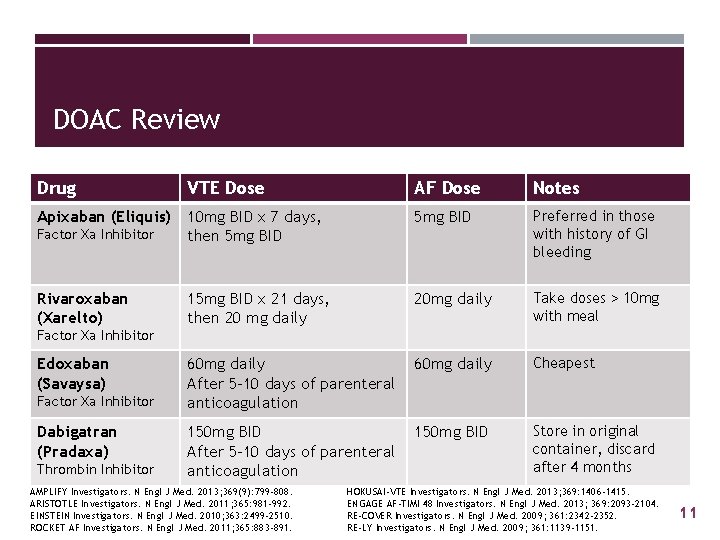
DOAC Review Drug VTE Dose AF Dose Notes Apixaban (Eliquis) 5 mg BID Factor Xa Inhibitor 10 mg BID x 7 days, then 5 mg BID Preferred in those with history of GI bleeding Rivaroxaban (Xarelto) 15 mg BID x 21 days, then 20 mg daily 20 mg daily Take doses > 10 mg with meal 60 mg daily After 5 -10 days of parenteral anticoagulation 60 mg daily Cheapest 150 mg BID After 5 -10 days of parenteral anticoagulation 150 mg BID Store in original container, discard after 4 months Factor Xa Inhibitor Edoxaban (Savaysa) Factor Xa Inhibitor Dabigatran (Pradaxa) Thrombin Inhibitor AMPLIFY Investigators. N Engl J Med. 2013; 369(9): 799 -808. ARISTOTLE Investigators. N Engl J Med. 2011; 365: 981 -992. EINSTEIN Investigators. N Engl J Med. 2010; 363: 2499 -2510. ROCKET AF Investigators. N Engl J Med. 2011; 365: 883 -891. HOKUSAI-VTE Investigators. N Engl J Med. 2013; 369: 1406 -1415. ENGAGE AF-TIMI 48 Investigators. N Engl J Med. 2013; 369: 2093 -2104. RE-COVER Investigators. N Engl J Med. 2009; 361: 2342 -2352. RE-LY Investigators. N Engl J Med. 2009; 361: 1139 -1151. 11

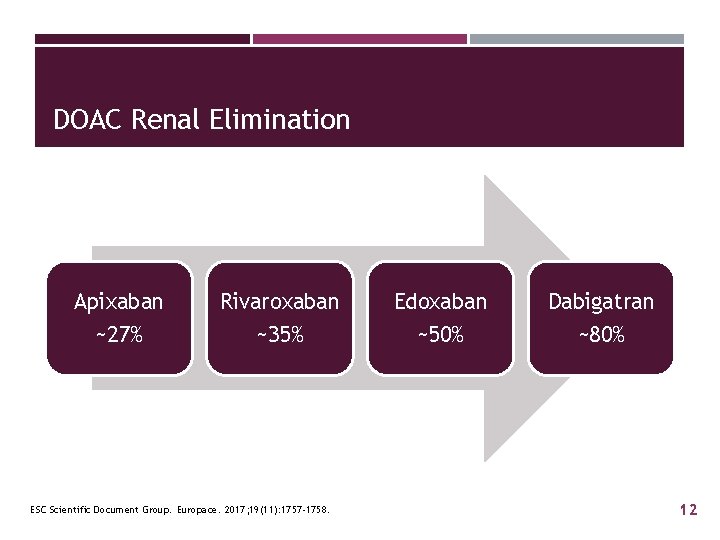
DOAC Renal Elimination Apixaban ~27% Rivaroxaban ~35% ESC Scientific Document Group. Europace. 2017; 19(11): 1757 -1758. Edoxaban ~50% Dabigatran ~80% 12

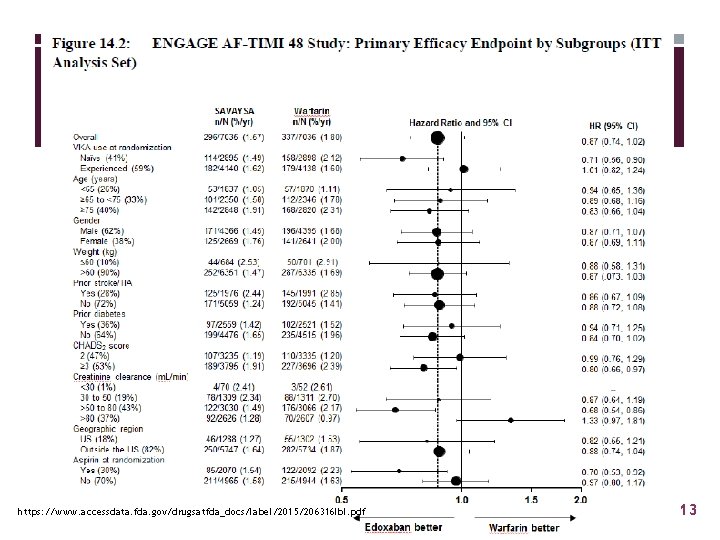
Edoxaban Contraindicated in patients with AF if Cr. Cl > 95 ml/min https: //www. accessdata. fda. gov/drugsatfda_docs/label/2015/206316 lbl. pdf 13

Dabigatran Dyspepsia AUC decreases by ~30% in patients taking a PPI No impact on clinical outcomes? Compliance issues – must store in original container Reversal agent https: //www. accessdata. fda. gov/drugsatfda_docs/label/2011/022512 s 007 lbl. pdf 14

Rivaroxaban Take doses > 10 mg with evening meal Increased absorption https: //www. accessdata. fda. gov/drugsatfda_docs/label/2011/202439 s 001 lbl. pdf 15

Apixaban Recommended in patients at increased risk for bleeding Prior history of GIB On DAPT Age > 75 Clinical judgement https: //www. accessdata. fda. gov/drugsatfda_docs/label/2012/202155 s 000 lbl. pdf 16

DOAC Effect on INR not monitored if on DOAC therapy Can increase INR, though not a safety or efficacy measure 17

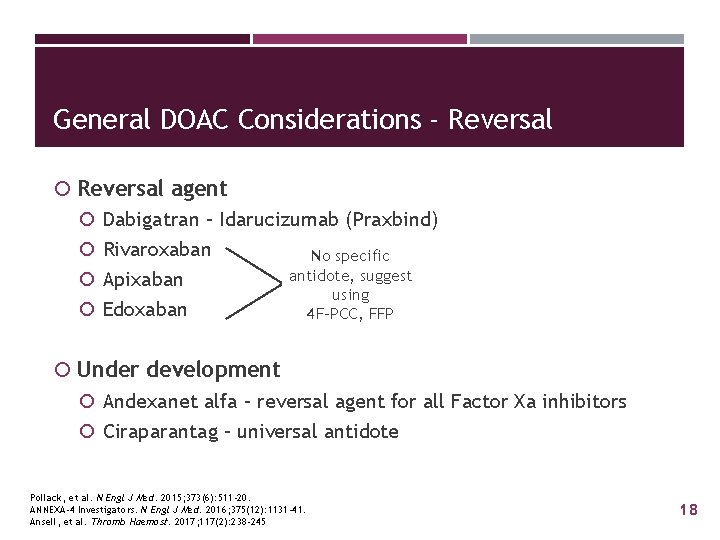
General DOAC Considerations - Reversal agent Dabigatran – Idarucizumab (Praxbind) Rivaroxaban No specific antidote, suggest Apixaban using Edoxaban 4 F-PCC, FFP Under development Andexanet alfa – reversal agent for all Factor Xa inhibitors Ciraparantag – universal antidote Pollack, et al. N Engl J Med. 2015; 373(6): 511 -20. ANNEXA-4 Investigators. N Engl J Med. 2016; 375(12): 1131 -41. Ansell, et al. Thromb Haemost. 2017; 117(2): 238 -245 18

General DOAC Considerations - Obesity Not recommended The ISTH 2016 guideline suggests avoiding the use of direct oral anticoagulants in patients with a BMI > 40 kg/m 2 or weight > 120 kg due to the lack of clinical data in this population. Subgroup analysis of obese patients from the pivotal phase 3 DOAC trials suggests that DOACs generally appear to be safe and effective; however, data are limited. Martin, et al. J Thromb Haemost. 2016; 14(6): 1308 -13. https: //www. pbm. va. gov/apps/VANational. Formulary/Get. File. aspx 19

General DOAC Considerations - Obesity VA PBM recommends that when a DOAC is being considered in such patients: “A shared decision making approach should be utilized with information provided on the limited data regarding the efficacy and safety of these agents in extremes of body weight and recommendations of some groups against use in this situation. " 20

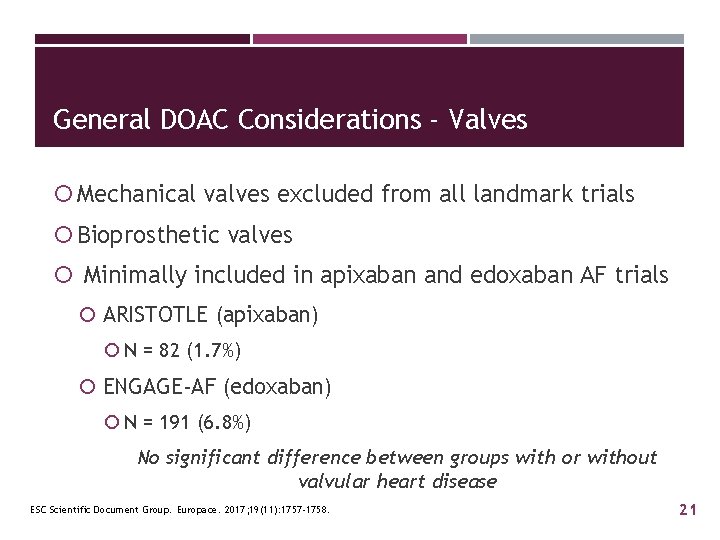
General DOAC Considerations - Valves Mechanical valves excluded from all landmark trials Bioprosthetic valves Minimally included in apixaban and edoxaban AF trials ARISTOTLE (apixaban) N = 82 (1. 7%) ENGAGE-AF (edoxaban) N = 191 (6. 8%) No significant difference between groups with or without valvular heart disease ESC Scientific Document Group. Europace. 2017; 19(11): 1757 -1758. 21

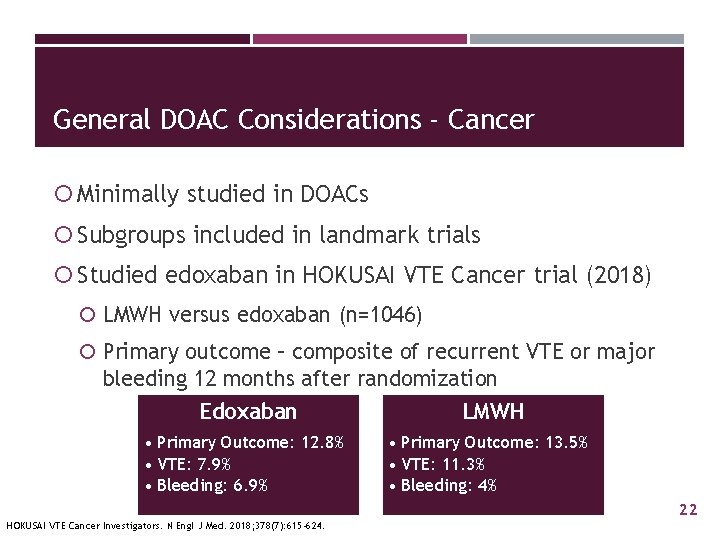
General DOAC Considerations - Cancer Minimally studied in DOACs Subgroups included in landmark trials Studied edoxaban in HOKUSAI VTE Cancer trial (2018) LMWH versus edoxaban (n=1046) Primary outcome – composite of recurrent VTE or major bleeding 12 months after randomization Edoxaban LMWH • Primary Outcome: 12. 8% • VTE: 7. 9% • Bleeding: 6. 9% • Primary Outcome: 13. 5% • VTE: 11. 3% • Bleeding: 4% 22 HOKUSAI VTE Cancer Investigators. N Engl J Med. 2018; 378(7): 615 -624.

RE-VERSE AD STUDY IDARUCIZUMAB (PRAXBIND) 23

RE-VERSE AD Multicenter, prospective, observational trial Sponsored by the Boehringer Ingelheim Objective Examine the efficacy and safety of idarucizumab for the reversal of the anticoagulant effects of dabigatran Idarucizumab is a monoclonal antibody fragment that binds dabigatran with an affinity that is 350 times as high as that observed with thrombin Pollack, et al. N Engl J Med. 2015; 373(6): 511 -20. 24

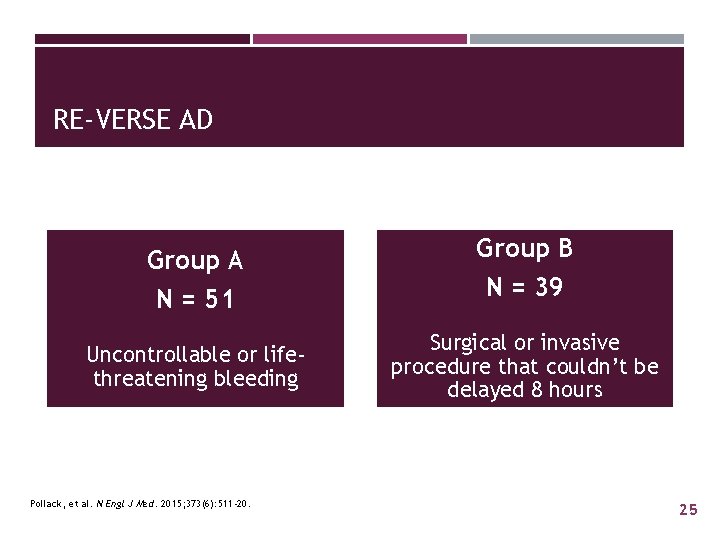
RE-VERSE AD Group A N = 51 Uncontrollable or lifethreatening bleeding Pollack, et al. N Engl J Med. 2015; 373(6): 511 -20. Group B N = 39 Surgical or invasive procedure that couldn’t be delayed 8 hours 25

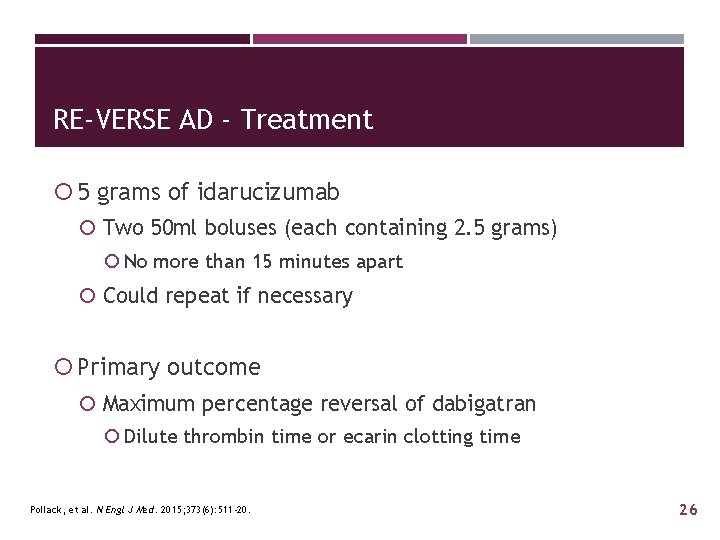
RE-VERSE AD - Treatment 5 grams of idarucizumab Two 50 ml boluses (each containing 2. 5 grams) No more than 15 minutes apart Could repeat if necessary Primary outcome Maximum percentage reversal of dabigatran Dilute thrombin time or ecarin clotting time Pollack, et al. N Engl J Med. 2015; 373(6): 511 -20. 26

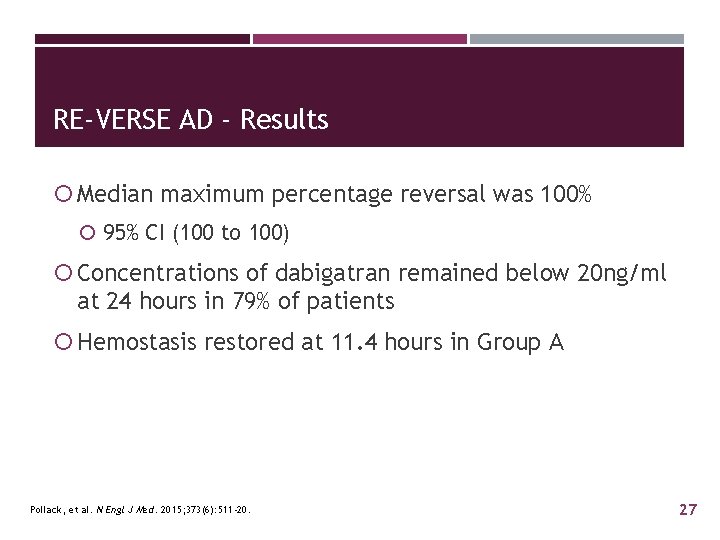
RE-VERSE AD - Results Median maximum percentage reversal was 100% 95% CI (100 to 100) Concentrations of dabigatran remained below 20 ng/ml at 24 hours in 79% of patients Hemostasis restored at 11. 4 hours in Group A Pollack, et al. N Engl J Med. 2015; 373(6): 511 -20. 27

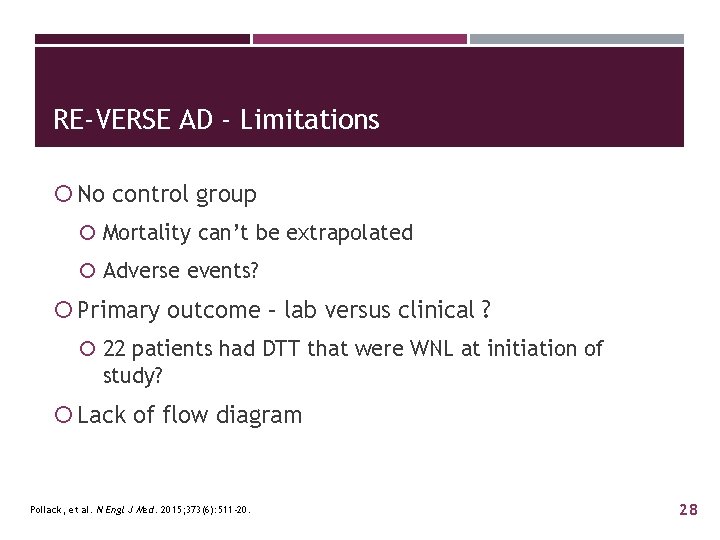
RE-VERSE AD - Limitations No control group Mortality can’t be extrapolated Adverse events? Primary outcome – lab versus clinical ? 22 patients had DTT that were WNL at initiation of study? Lack of flow diagram Pollack, et al. N Engl J Med. 2015; 373(6): 511 -20. 28

Bottom Line DOACs can be complicated Don’t take studies at face value Perform thorough histories to determine appropriate agents Utilize pharmacy staff 29