Anticoagulant Antiplatelet and Fibrinolytic Drugs Thrombosis the formation

Anticoagulant, Antiplatelet and Fibrinolytic Drugs

Thrombosis, the formation of unwanted clot within a blood vessel, is the most common abnormality of hemostasis. Thromboembolic disorders include acute MI, DVT, pulmonary embolism, acute ischemic stroke. Bleeding disorders, such as hemophilia, are less common than thromboembolic diseases. A clot that adheres to a vessel wall is called a thrombus, whereas an intravascular clot that floats in the blood is called an embolus. Arterial thrombosis most often occurs in medium-sized vessels, and usually consists of a platelet-rich clot. Venous thrombosis, a clot rich in fibrin, is triggered by blood stasis or abnormal activation of coagulation cascade. 2
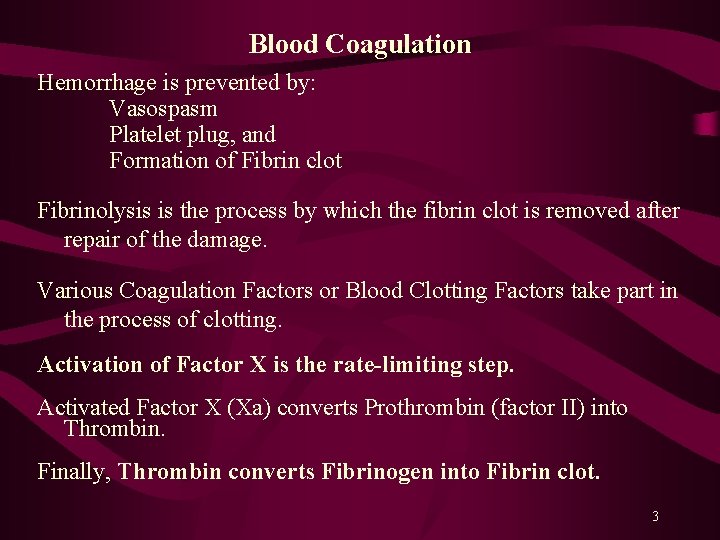
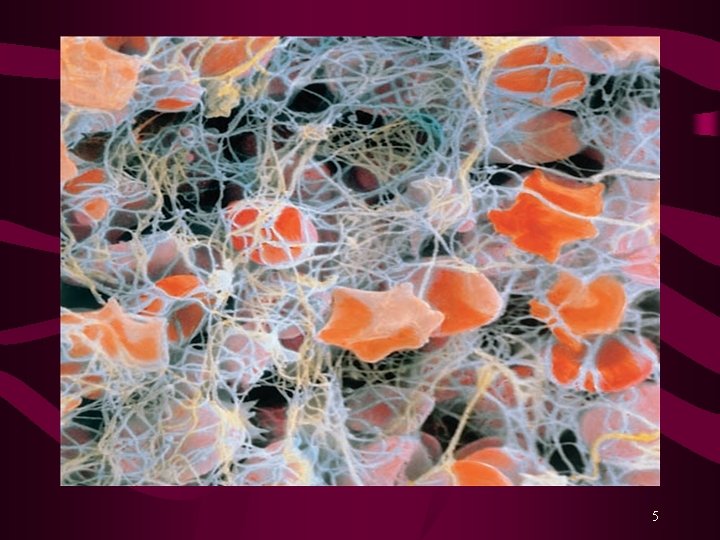
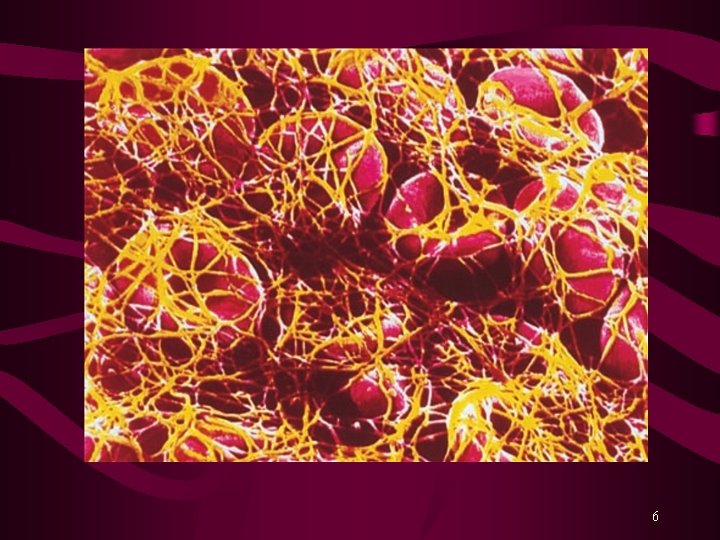
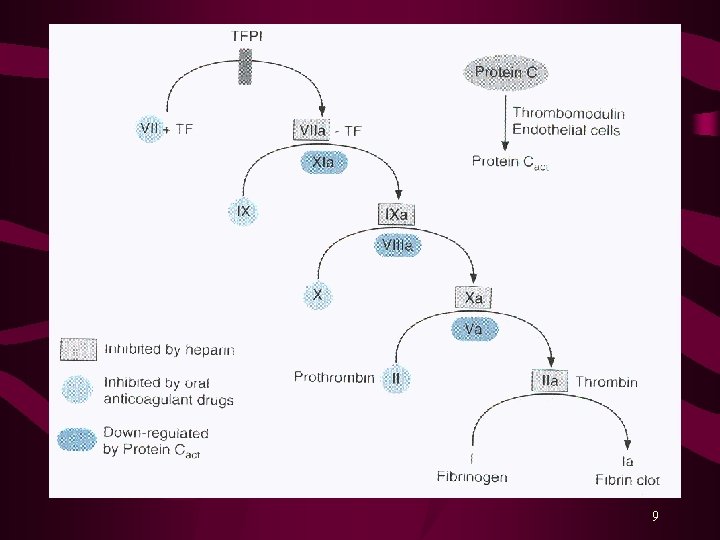
Blood Coagulation Hemorrhage is prevented by: Vasospasm Platelet plug, and Formation of Fibrin clot Fibrinolysis is the process by which the fibrin clot is removed after repair of the damage. Various Coagulation Factors or Blood Clotting Factors take part in the process of clotting. Activation of Factor X is the rate-limiting step. Activated Factor X (Xa) converts Prothrombin (factor II) into Thrombin. Finally, Thrombin converts Fibrinogen into Fibrin clot. 3
4
5
6

Clotting Factors and Drugs Affecting Them Blocked by Factor I II IV V VIII IX X XI XIII Fibrinogen Prothrombin* Heparin, Warfarin Tissue Thromboplastin Calcium Proaccelerin Proconvertin* Warfarin Antihemophilic factor Christmas factor* Warfarin Stuart-Power factor* Heparin, Warfarin Plasma thromboplastin antecedent Hageman factor Fibrin-stabilizing factor *Vitamin K-dependent 7

Regulation of Coagulation and Fibrinolysis Two major systems regulate and delineate these processes. Fibrin Inhibition Fibrinolysis Plasma contains protease inhibitors that rapidly inactivate coagulation proteins as they escape from the injured site. Most important proteins of this system are: 1 -Antiprotease 2 -Macroglobulin 2 -Antiplasmin Antithrombin Failure of this system may result in Disseminated Intravascular Coagulation (DIC), such as in cases of massive tissue injury, cell lysis in malignancy, obstetric emergencies or bacterial sepsis. 8
9

ANTICOAGULANT DRUGS Anticoagulant drugs retard coagulation and prevent the occurrence or enlargement of a thrombus. There are four classes of anticoagulant drugs: I. Oral anticoagulants, e. g. Warfarin II. Unfractionated and LMW Heparins III. Direct Thrombin Inhibitors, e. g. Lepirudin, Bivalirudin, Argatroban, and Dabigatran IV. Selective Factor Xa Inhibitors, e. g. Fondaparinux Recombinant Activated Protein C (r-APC) has also been approved by the FDA for patient with severe sepsis and evidence of acute organ dysfunction, shock, oliguria 10

Oral Anticoagulants Warfarin (Coumadin. R) Dicumarol, and Anisindione Coumarins were 1 st identified in spoiled sweet clover hay and identified as bishydroxycoumarin and synthesized as Dicumarol. Warfarin is a potent synthetic congener of Dicumarol and is the most reliable member of this group. Other coumarin anticoagulants are almost never used in the U. S. A. Dicumarol and Warfarin were introduced in 1940 s as rodenticide and as oral anticoagulants. 11

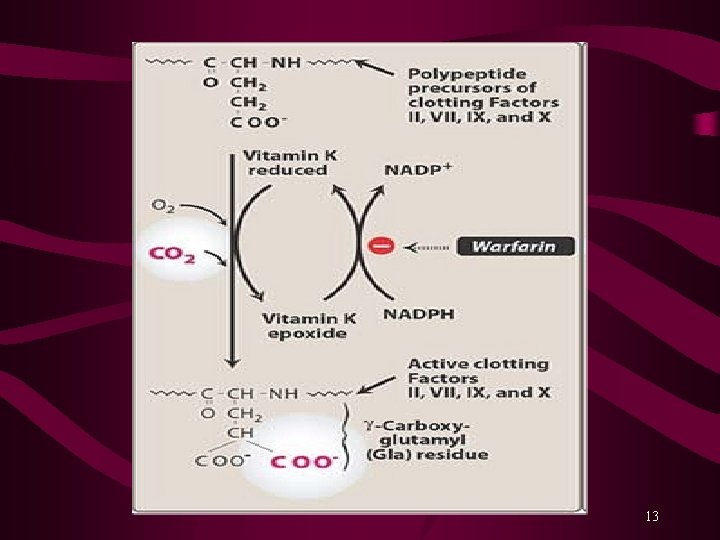
Mechanism of Action Warfarin and Dicumarol are structurally related to Vitamin K and inhibit synthesis of clotting factors II, VII, IX and X. Warfarin blocks the epoxide reductase that mediates regeneration of reduced Vit. K. Depletion of reduced Vit. K in the liver prevents the γ-carboxylation reaction, required for the synthesis of active coagulation factors. Oral anticoagulant have a delay in the onset of action (8 -12 hours) due to the time required to deplete the pool of circulating clotting factors. Maximum effect of oral anticoagulants is not observed until 3 -5 days after starting therapy. Several days are also required for these factors to return to normal after the oral anticoagulant therapy is discontinued. 12
13

Pharmacokinetics Orally administered Warfarin is nearly 100% bioavailable. Plasma level peaks at 0. 5 to 4 hours after administration. In the plasma, 99% of racemic warfarin is bound to plasma protein (albumin) Warfarin has a long elimination T 1/2 of 36 hours. It is hydroxylated by CYP 450 system to inactive metabolites and excreted in the urine. It has a narrow therapeutic index (TI) and has potential for many drug-drug interactions; thus its levels are closely monitored. 14
Indications and Clinical Uses Long-term management of patients with thromboembolic disorders, such as Deep Vein Thrombosis (DVT) or Atrial Fibrillation Patients with Artificial Heart Valve In conjunction with Heparin for the treatment of MI. Goal of Therapy: To inhibit embolization and prevent serious and potentially fatal complications of thrombosis. Oral anticoagulants prevent extension of established thrombus but they cannot dissolve it. Prothrombin Time (PT): Oral anticoagulant therapy is closely monitored and adjusted based on PT value. 15

Adverse Effects Bleeding: From mild nosebleed to life-threatening hemorrhage Contraindications Active or past G. I. ulceration Aneurysm Bacterial endocarditis Bleeding Chronic alcoholism Hepatic or renal disease Hypersensitivity Recent brain, eye or spinal cord Surgery Pregnancy: Crosses placenta, causes fetal hemorrhage and structural malformations (including abnormal bone formation), known as Fetal Warfarin Syndrome. Warfarin should never be administered during pregnancy. Phytonadione (Vit. K), fresh frozen plasma, prothrombin complex concentration are used to reverse excessive anticoagulant effect. 16

Drug-Drug Interactions Pharmacokinetic Interactions Induction of CYP 450 (Rifampin, Barbiturates and others) Inhibition of CYP 450 (Amiodarone, Cimetidine, Fluconazole, Metronidazole, Chloramphenicol, Reduced Plasma Protein Binding (Phenylbutazone, Sulfinpyrazone) Cholestyramine inhibits absorption of Warfarin. Pharmacodynamic Interactions Synergism (Drugs: Aspirin, 3 rd Gen. Cephalosporins, Heparin; Diseases: Hepatic disease, Hyperthyroidism) Competitive Antagonism (Vit. K), Hypothyroidism 17

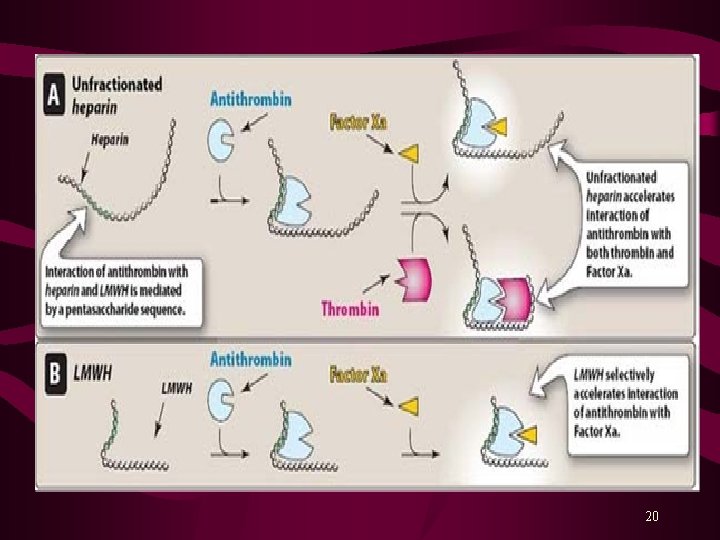
Indirect Thrombin Inhibitors Parenteral Anticoagulants Unfractionated Heparin LMW Heparins; Enoxaparin, Dalteparin and Tinzaparin Heparin is a mixture of sulfated mucopolysaccharides, stored in secretory granules of mast cells and basophils. Heparin molecules are highly negatively charged and endogenous heparin is the strongest organic acid in the body. Heparin contains fractions with high molecular weight and low molecular weight. Enoxaparin (Lovenox. R), Dalteparin (Fragmin. R) & Tinzaparin (Innohep. R) are LMW forms of fractionated heparin. 18

Mechanism of Action Heparin inactivates clotting factors. Predominantly potentiates activity of an endogenous anticoagulant called, Antithrombin III (ATIII). ATIII then inactivates Thrombin (factor IIa) and other clotting factors. Enoxaparin, Dalteparin and Tinzaparin directly inactivate factor Xa, responsible for the conversion of Prothrombin to Thrombin. Heparin is not absorbed from gut and is administered by continuous I. V. infusion. Enoxaparin, Dalteparin and Tinzaparin are given s. c. , and their maximal effect occurs from 3 -5 hours after the injection. 19
20
Indications and Clinical Uses Treatment of Acute Thromboembolic Disorders, such as: Peripheral and Pulmonary Embolism MI, Venous Thrombosis Disseminated Intravascular Coagulation (DIC) Prophylactically to prevent clotting in: Arterial and Heart Surgery, Heart Valve replacement Blood Transfusions Renal Dialysis Cardiac Arrhythmias, primarily Atrial Fibrillation Deep Vein Thrombosis (DVT) in post-operative patients Pulmonary Embolism Enoxaparin, Dalteparin and Tinzaparin can be given s. c. to prevent DVT in patients with hip replacement surgery. Protamine sulfate is the specific antagonist of Heparin (1 mg/100 u) 21

Adverse Effects Bleeding: Elderly women and patients with renal failure more prone. Thrombocytopenia (Type 1 within 5 days; Type 2 between 5 -14 days) Hyperkalemia by Heparin due to suppression of Aldosterone Allergic reactions Reversible alopecia Osteoporosis and fractures on long-term use Spinal or Epidural hematomas with LMW heparins when used during spinal/epidural anesthesia or spinal puncture Contraindications Hypersensitivity to Heparin Active bleeding and Hemophilia Significant Thrombocytopenia Purpura Severe Hypertension, Intracranial Hemorrhage, Infective Carditis Active Tuberculosis, Advanced Hepatic or Renal disease Ulcerative lesions of G. I. T. Threatened Abortion, Patients who had brain, spinal cord or eye surgery 22

Direct Thrombin Inhibitors Hirudin, Lepirudin, Bivalirudin and Argatroban Hirudin is obtained from the leech and is a direct thrombin inhibitor. Lepirudin is produced by r. DNA technology from Hirudin. One molecule of Lepirudin binds to one molecule of thrombin, blocking the thrombin -mediated activation of fibrinogen and factor XIII. Highly effective because it inhibits both free and fibrin-bound thrombin in developing clots; and its binding to thrombin is essentially irreversible. Lepirudin has a half-life of about one hour, and is administered I. V. Uses: Especially in patients who suffer from heparin-induced thrombocytopenia. 50% patients develop antibodies to Lepirudin, and the drug-antibody complex retains anticoagulant activity. A/Es: Bleeding; Clotting time must be monitored. 23

Bivalirudin (Angiomax. TM) is a synthetic peptide that binds to thrombin and inhibits thrombin-mediated activation of fibrinogen and factor XIII. It is excreted renally and has a short half-life of 25 min. Uses: Anticoagulation in patients undergoing angiography or angioplasty. Argatroban is a small molecule direct inhibitor of thrombin. Unlike other direct thrombin inhibitors it is excreted by biliary secretion. It can be used in patients with renal insufficiency. Uses: For patients with heparin-induced thrombocytopenia. 24

DABIGATRAN (Pradaxa™) Dabigatran is a new oral anticoagulant that directly inhibits thrombin. Unlike Warfarin, Dabigatran does not require periodic monitoring of PT FDA approved it on Oct. 19, 2010 for use in patients with non-valvular atrial fibrillation to reduce risk of stroke and systemic embolism. A/Es: Bleeding, sometimes life-threatening and fatal. Other side effects include dyspepsia, stomach pain, nausea, heartburn and bloating. Once a bottle of Dabigatran is opened, the medication is considered to be expired after only thirty days. 25

Selective Factor Xa Inhibitor Fondaparinux (Arixtra. TM) A synthetic pentasaccharide, containing sequence of five essential carbohydrates necessary for binding to antithrombin III. MOA: It induces a conformational change in antithrombin III that is required for conjugation to factor Xa. Specific inhibitor of Xa, with negligible antithrombin activity. It is excreted renally and is used as a daily single s. c. injection. Clinical Uses: Prevention and Treatment of DVT and pulmonary embolism in patients undergoing hip or knee surgery Contraindicated: Renal insufficiency (excreted renally) 26

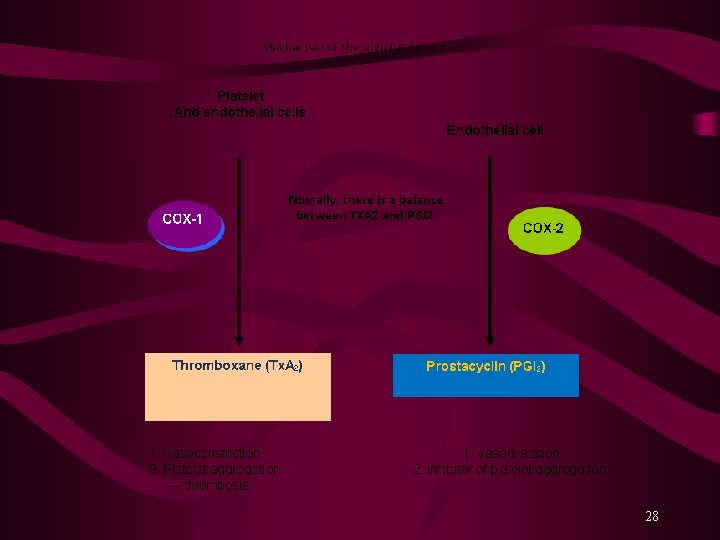
Antiplatelet Drugs Normal endothelium releases prostacycline (PG I 2) and NO. PG I 2 binds to its receptors on platelets’ surface and stimulates synthesis of intracellular c. AMP that lowers free intracellular Ca 2+ level and inhibits platelet aggregation. Platelets also contain receptors for thrombin, thromboxanes and exposed collagen. Endothelium injury exposes subendothelial collagen, platelets bind to it and are activated, releasing chemical mediators, such as ADP, TXA 2, 5 -HT, PAF and thrombin. These molecules signal to other platelets, causing conformational changes that activate them, causing platelets aggregation. 27
28

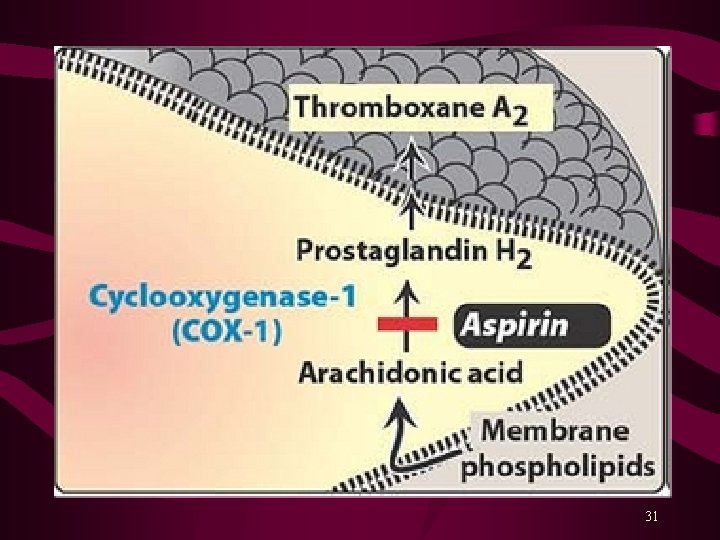
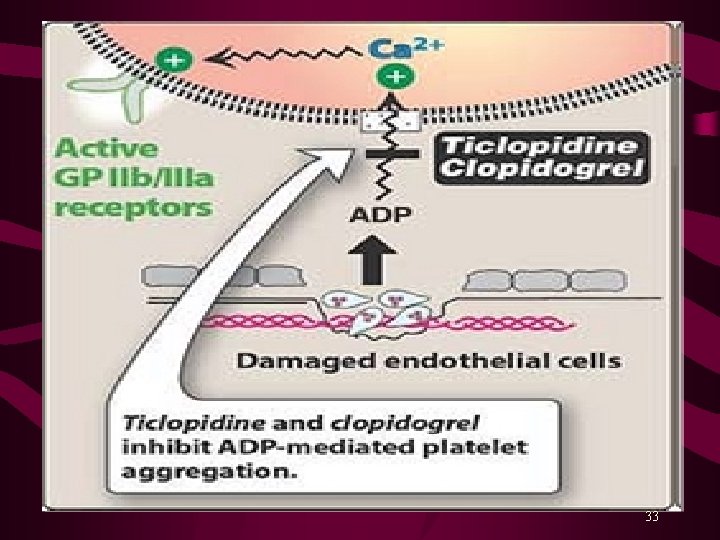
Platelet aggregation results due to ↑ intracellular Ca 2+ and a ↓ c. AMP level within platelets. Activation of glycoprotein (GP) IIb/IIIa receptors on platelets’ surface bind fibrinogen* and platelet-platelet interaction. Aspirin inhibits synthesis of prostaglandins from arachidonic acid. Ticlopidine (Ticlid. R) and Clopidogrel (Plavix. R) inhibit ADP-induced platelet aggregation. Abciximab is a monoclonal antibody binds to platelet GPIIb/IIIa receptors and prevents binding of fibrinogen and other adhesive molecules. Tirofiban and Eptifibatide are competitive, reversible inhibitors of fibrinogen binding to GPIIb/IIIa receptors. Dipyridamole is a PDE inhibitor, a coronary vasodilator, and a weak antiplatelet aggregator. 29
Aspirin is an NSAID, with analgesic, antipyretic and antiinflammatory activities. Low doses of aspirin selectively inhibits synthesis of thromboxane (TXA 2) without significantly affecting synthesis of Prostacyclin. Aspirin irreversibly inhibits cyclooxygenase (COX) enzyme for the life of platelets and effectively reduces platelet aggregation when used once daily or every other day. Indications and Clinical Uses Prevention of arterial thrombosis in patients with: Ischemic Heart Disease and Stroke (MI in patients with) Unstable Angina Transient Ischemic Attacks (TIAs) Artificial Heart Valves 30
31
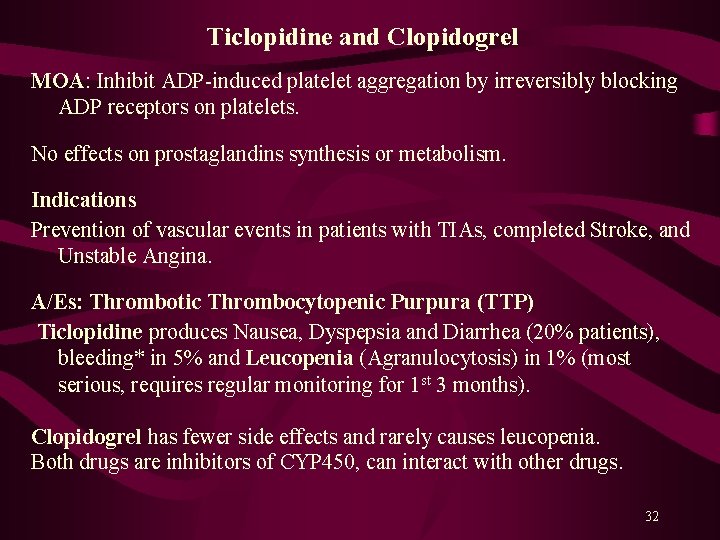
Ticlopidine and Clopidogrel MOA: Inhibit ADP-induced platelet aggregation by irreversibly blocking ADP receptors on platelets. No effects on prostaglandins synthesis or metabolism. Indications Prevention of vascular events in patients with TIAs, completed Stroke, and Unstable Angina. A/Es: Thrombotic Thrombocytopenic Purpura (TTP) Ticlopidine produces Nausea, Dyspepsia and Diarrhea (20% patients), bleeding* in 5% and Leucopenia (Agranulocytosis) in 1% (most serious, requires regular monitoring for 1 st 3 months). Clopidogrel has fewer side effects and rarely causes leucopenia. Both drugs are inhibitors of CYP 450, can interact with other drugs. 32
33
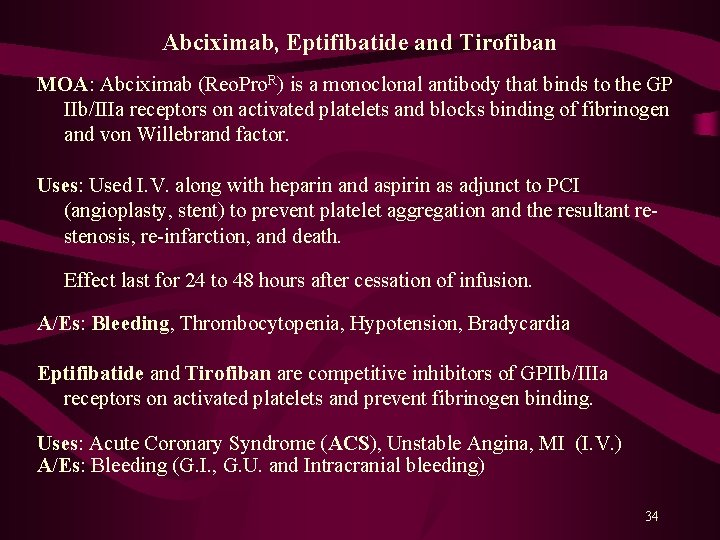
Abciximab, Eptifibatide and Tirofiban MOA: Abciximab (Reo. Pro. R) is a monoclonal antibody that binds to the GP IIb/IIIa receptors on activated platelets and blocks binding of fibrinogen and von Willebrand factor. Uses: Used I. V. along with heparin and aspirin as adjunct to PCI (angioplasty, stent) to prevent platelet aggregation and the resultant restenosis, re-infarction, and death. Effect last for 24 to 48 hours after cessation of infusion. A/Es: Bleeding, Thrombocytopenia, Hypotension, Bradycardia Eptifibatide and Tirofiban are competitive inhibitors of GPIIb/IIIa receptors on activated platelets and prevent fibrinogen binding. Uses: Acute Coronary Syndrome (ACS), Unstable Angina, MI (I. V. ) A/Es: Bleeding (G. I. , G. U. and Intracranial bleeding) 34

Phosphodiesterase Inhibitors Dipyridamole and Cilostazol Both inhibit PDE resulting in ↑ in intracellular concentration of c. AMP that leads to ↓ in platelet aggregation. Platelet c. AMP levels are regulated by Tx. A 2 and PGI 2. Inhibitors of platelet PDE ↓ platelet aggregation by inhibiting c. AMP degradation, while activators of platelet AC ↓ platelet aggregability by increasing c. AMP synthesis. Dipyridamole is a PDE inhibitor and has weak antiplatelet effects. It also possesses vasodilatory activity; may cause “Coronary Steal” Usually used with Aspirin or Warfarin for thrombotic diathesis. 35

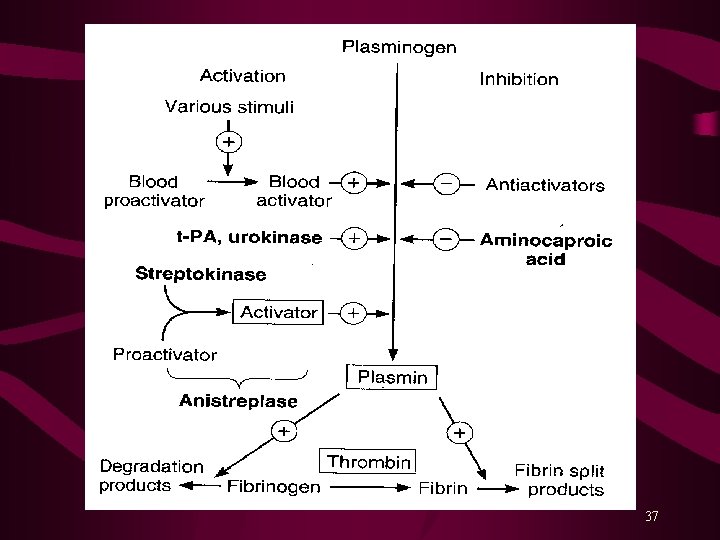
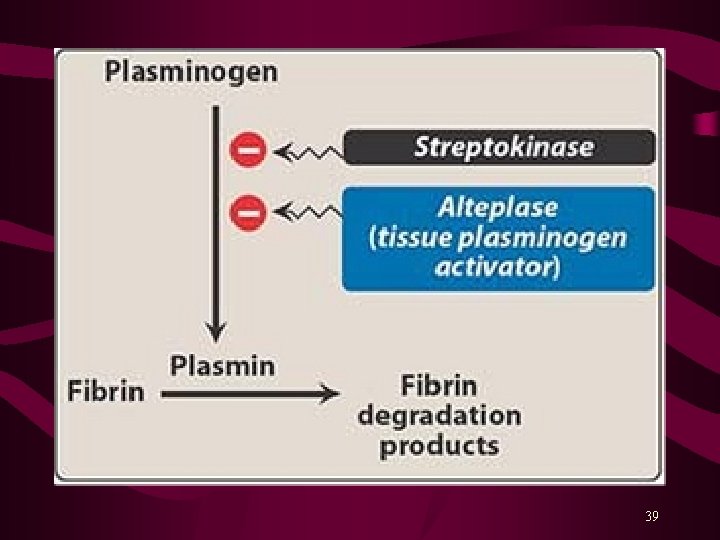
Fibrinolytic or Thrombolytic Drugs Fibrinolytic drugs rapidly lyse thrombi by catalyzing formation of Plasmin from plasminogen*. Thrombolytics should be used within a 3 hours after the onset of symptoms to limit the infarct size. Fibrinolytic drugs form plasmin inside thrombus where it is protected from plasma antiplasmin. These drugs produce generalized lysis & break down both protective and targeted thrombi. Alteplase (rh-t. PA) clot-specific, shorter T 1/2 Reteplase (rh-t. PA) < fibrin-specific than Alteplase, longer T 1/2 Tenecteplase (rh-t. PA) longer T 1/2 (Bolus inj. ) Urokinase (an enzyme obtained from human urine) Streptokinase (a protein from β-hemolytic Streptococci) 36
37

Streptokinase combines with plasminogen to form an activator complex that converts free inactive plasminogen to plasmin. Streptokinase acts on both bound and free plasminogen, and is not clot specific. It also depletes circulating fibrinogen and factors V and VII. Streptokinase is antigenic; may cause hypersensitivity reactions, including anaphylaxis and hypotension. Streptococcal antibodies due to recent past infection or use may decrease its activity. Alteplase (t. PA) is clot-specific and acts mainly on fibrin-bound plasminogen; shorter half-life, requires adjunct use of Heparin. Alteplase is non-antigenic; more likely to cause intracranial hemorrhage. 38
39

Indications and Clinical Uses Multiple Pulmonary Emboli Central Deep Venous Thrombosis (DVT) Acute Myocardial Infarction (STEMI) Acute Ischemic (Thrombotic) Stroke Peripheral Vascular Disease (PVD) All fibrinolytic drugs are administered intravenously. Use of fibrinolytic drugs within 3 hours post MI decreases mortality by >60% (short half-life of about 5 min. ). Use of aspirin further decreases mortality and use of Adenosine reduces the infarct size. A/E*: Bleeding, Possible intracranial hemorrhage Hypersensitivity reactions with Streptokinase Antidotes: Aminocaproic acid/Tranexamic acid inhibit fibrinolysis 40

Inhibitors of Anticoagulation and Fibrinolysis Protamine: A low molecular weight polycationic protein, is a chemical antagonist of Heparin. MOA: Forms a stable complex with negatively charged heparin molecule. Uses: Administered IV to reverse the effects of unfractionated Heparin. Partially effective against LMW Heparins. Aprotinine: Inhibitor of Plasmin, , and Thrombin MOA: By inhibiting fibrinolysis, promotes clot stabilization. Inhibition of thrombin may also promote platelet activity. Uses: To Reverse fibrinolytic activity A/Es: Potential for postoperative acute renal failure 41

Aminocaproic Acid and Tranexamic Acid Analogues of lysine MOA: Bind to and inhibit Plasminogen and Plasmin Uses: To reduce perioperative bleeding during CABG surgery To counter the excessive effects of fibrinolytics Aprotinin is also used for the same purpose. Aminocaproic Acid and Tranexamic Acid do not produce post-operative acute renal failure. 42
- Slides: 42