Anticancer Agents Dr Manal Najdawi Part II Nitrogen








![Antimetabolites—— Folic Acid Antagonist Methotrxate L-(+)-N-[p[[2, 4 -diamino-6 pteridinyl)methyl]methylamino]-benzoyl]glutamic acid Folic acid Antimetabolites—— Folic Acid Antagonist Methotrxate L-(+)-N-[p[[2, 4 -diamino-6 pteridinyl)methyl]methylamino]-benzoyl]glutamic acid Folic acid](https://slidetodoc.com/presentation_image_h/d11c89e8d4481d8e2a3abeb978624807/image-29.jpg)

- Slides: 50

Anticancer Agents Dr. Manal Najdawi Part II

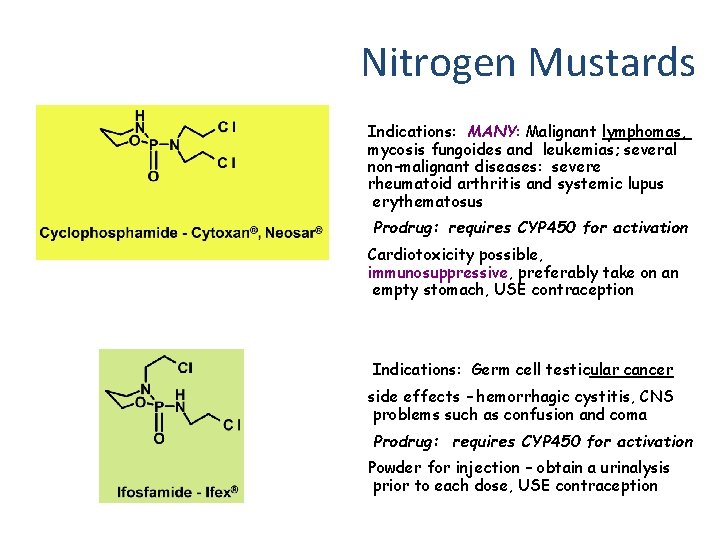
Nitrogen Mustards Indications: MANY: Malignant lymphomas, mycosis fungoides and leukemias; several non-malignant diseases: severe rheumatoid arthritis and systemic lupus erythematosus Prodrug: requires CYP 450 for activation Cardiotoxicity possible, immunosuppressive, preferably take on an empty stomach, USE contraception Indications: Germ cell testicular cancer side effects – hemorrhagic cystitis, CNS problems such as confusion and coma Prodrug: requires CYP 450 for activation Powder for injection – obtain a urinalysis prior to each dose, USE contraception

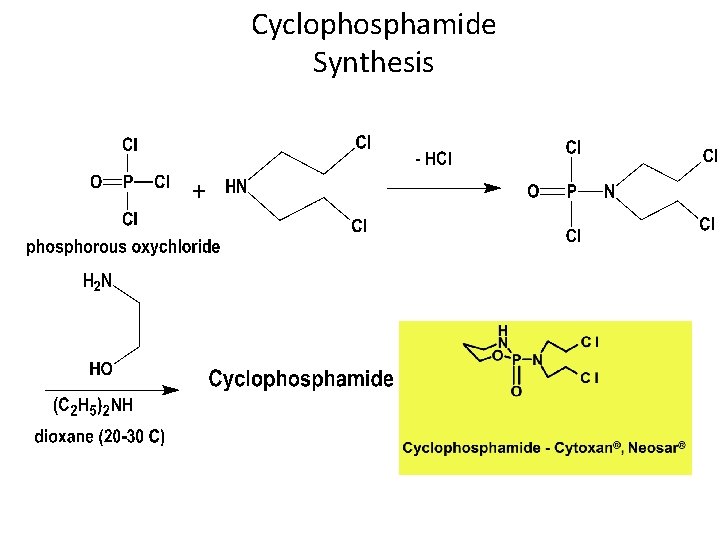
Cyclophosphamide Synthesis

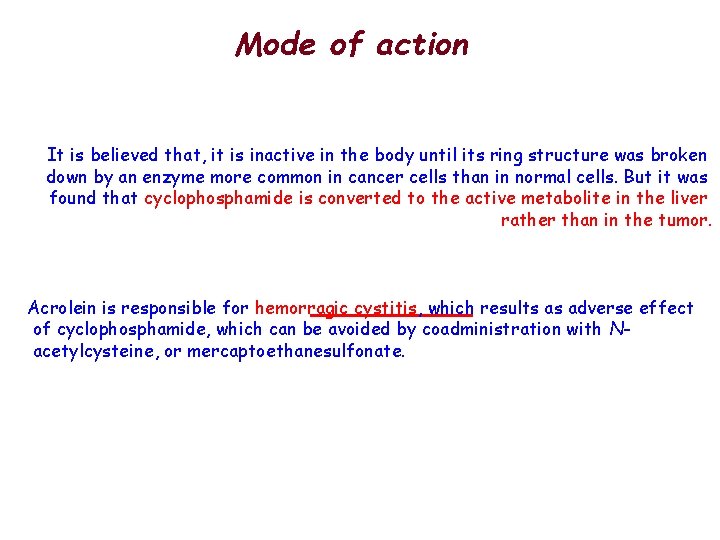
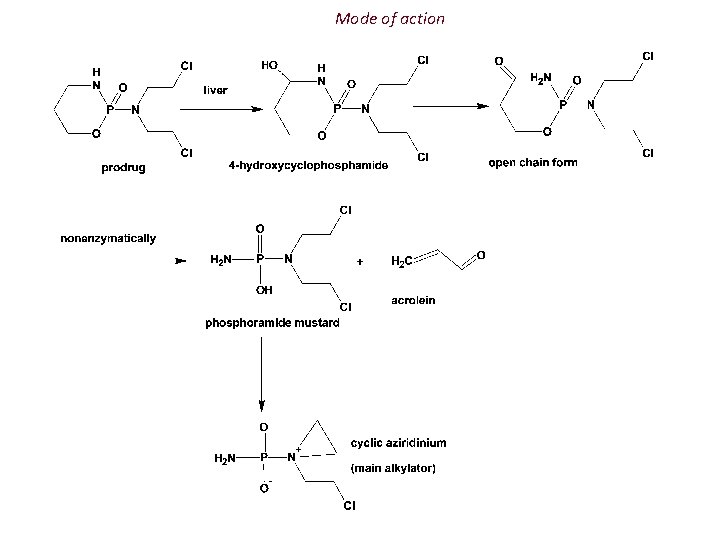
Mode of action It is believed that, it is inactive in the body until its ring structure was broken down by an enzyme more common in cancer cells than in normal cells. But it was found that cyclophosphamide is converted to the active metabolite in the liver rather than in the tumor. Acrolein is responsible for hemorragic cystitis, which results as adverse effect of cyclophosphamide, which can be avoided by coadministration with Nacetylcysteine, or mercaptoethanesulfonate.

Mode of action

Nitrosoureas Prototype Indications: Palliative treatment for brain tumors, multiple myeloma, Hodgkin’s and non-Hodgkin’s lymphomas Non-vesicant, I. V. or topical Highly lipid soluble (may cross BBB) 1, 3 -Bis(2 -chloroethyl)-1 -nitrosourea Long delay in bone marrow suppression (6 weeks) - do not give more often than every 6 weeks Indications: Brain tumors and Hodgkin’s disease Bone marrow toxicity is cumulative - delayed for 6 weeks Capsule: take on empty stomach to avoid N/V, avoid alcohol Highly lipid soluble allows 50% higher CNS levels 3 -Cyclohexyl-1 -(2 -chloroethyl)-1 nitrosourea

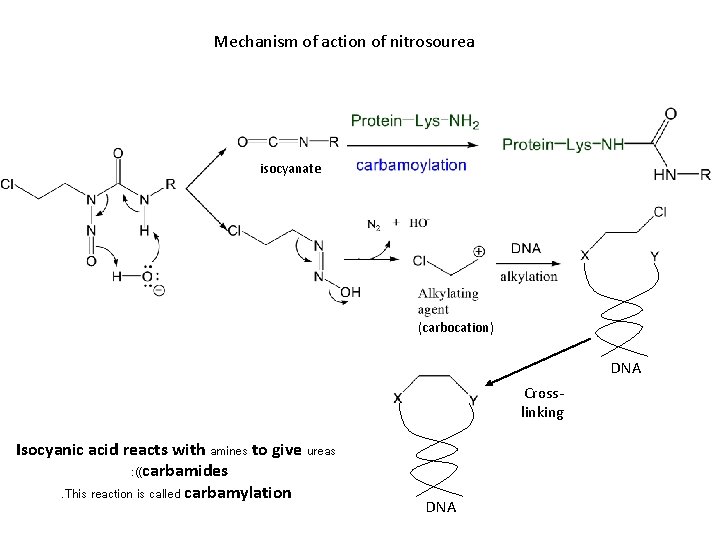
Mechanism of action of nitrosourea isocyanate (carbocation) DNA Crosslinking Isocyanic acid reacts with amines to give ureas : ((carbamides. This reaction is called carbamylation DNA

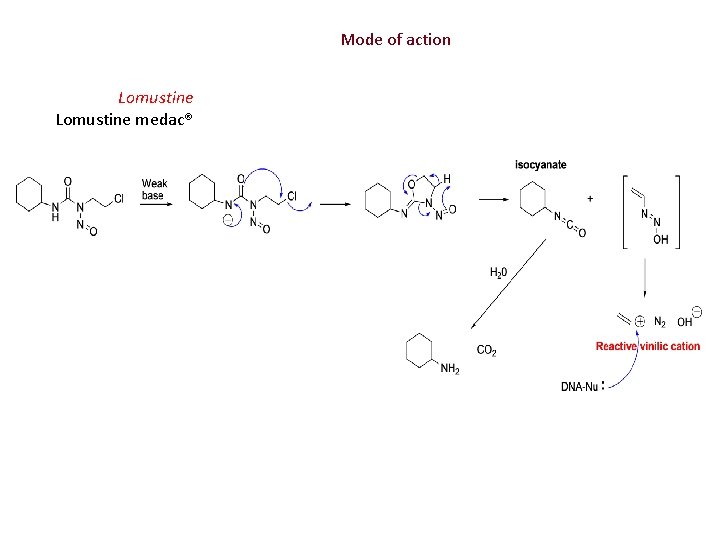
Mode of action Lomustine medac®

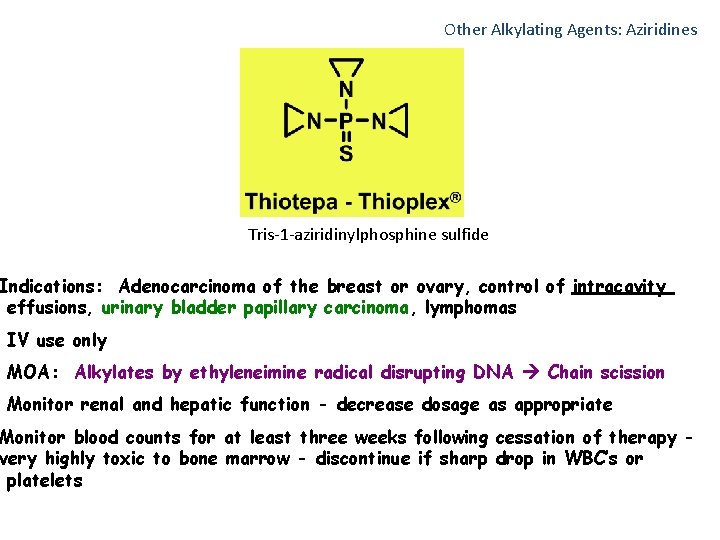
Other Alkylating Agents: Aziridines Tris-1 -aziridinylphosphine sulfide Indications: Adenocarcinoma of the breast or ovary, control of intracavity effusions, urinary bladder papillary carcinoma, lymphomas IV use only MOA: Alkylates by ethyleneimine radical disrupting DNA Chain scission Monitor renal and hepatic function - decrease dosage as appropriate Monitor blood counts for at least three weeks following cessation of therapy very highly toxic to bone marrow - discontinue if sharp drop in WBC’s or platelets

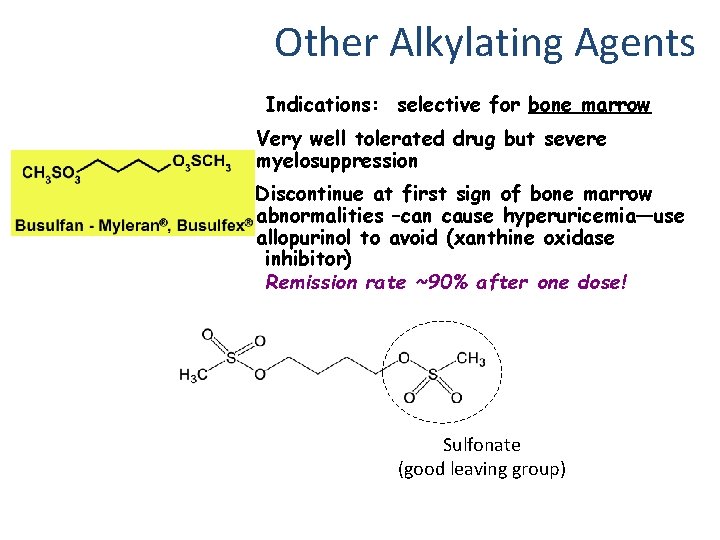
Other Alkylating Agents Indications: selective for bone marrow Very well tolerated drug but severe myelosuppression Discontinue at first sign of bone marrow abnormalities –can cause hyperuricemia—use allopurinol to avoid (xanthine oxidase inhibitor) Remission rate ~90% after one dose! Sulfonate (good leaving group)


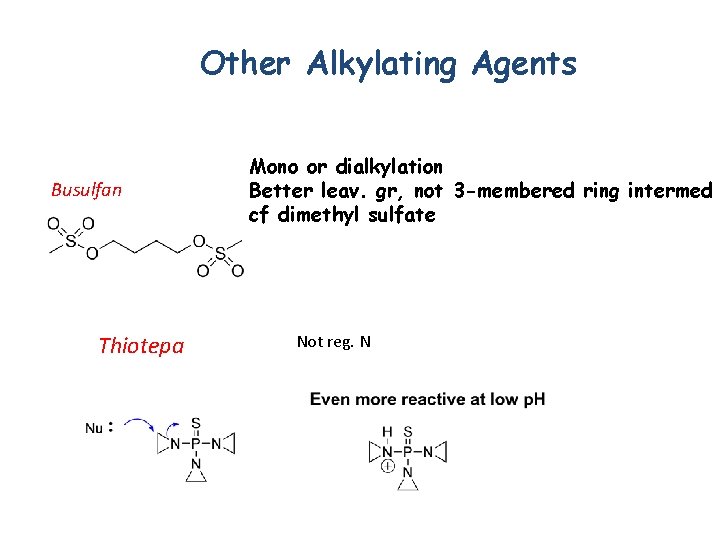
Other Alkylating Agents Busulfan Thiotepa Mono or dialkylation Better leav. gr, not 3 -membered ring intermed cf dimethyl sulfate Not reg. N

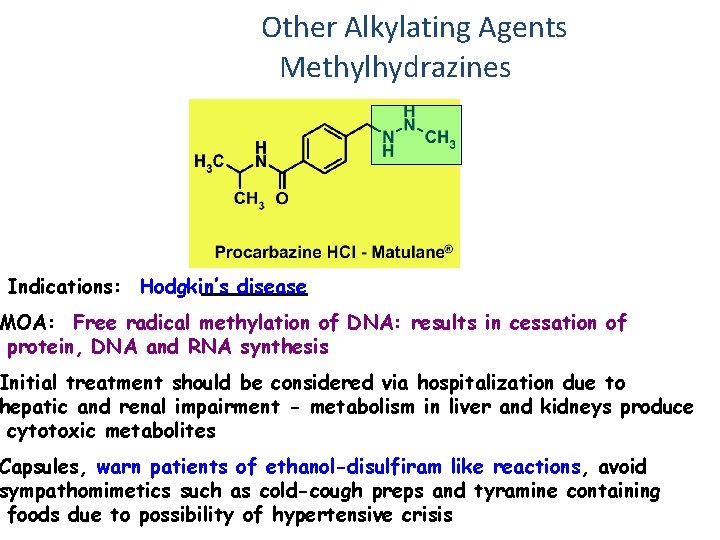
Other Alkylating Agents Methylhydrazines Indications: Hodgkin’s disease MOA: Free radical methylation of DNA: results in cessation of protein, DNA and RNA synthesis Initial treatment should be considered via hospitalization due to hepatic and renal impairment - metabolism in liver and kidneys produce cytotoxic metabolites Capsules, warn patients of ethanol-disulfiram like reactions, avoid sympathomimetics such as cold-cough preps and tyramine containing foods due to possibility of hypertensive crisis

Procarbazine Prodrug • CH 3. ) methyl group )methyl radical guanine base • •


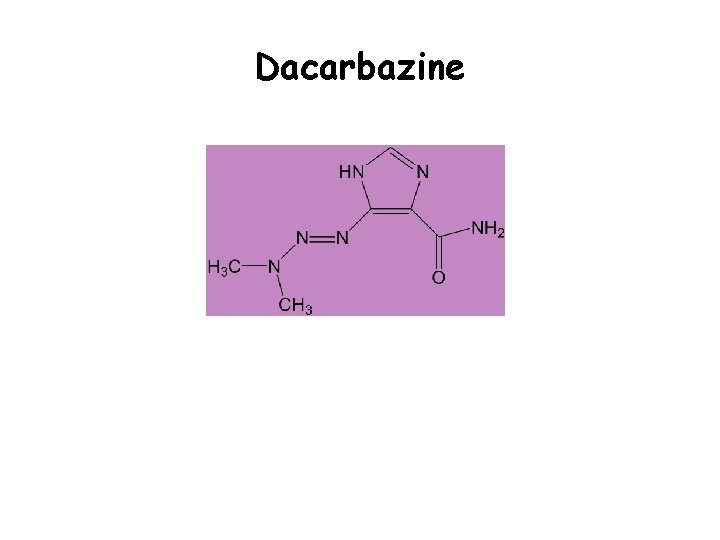
Dacarbazine


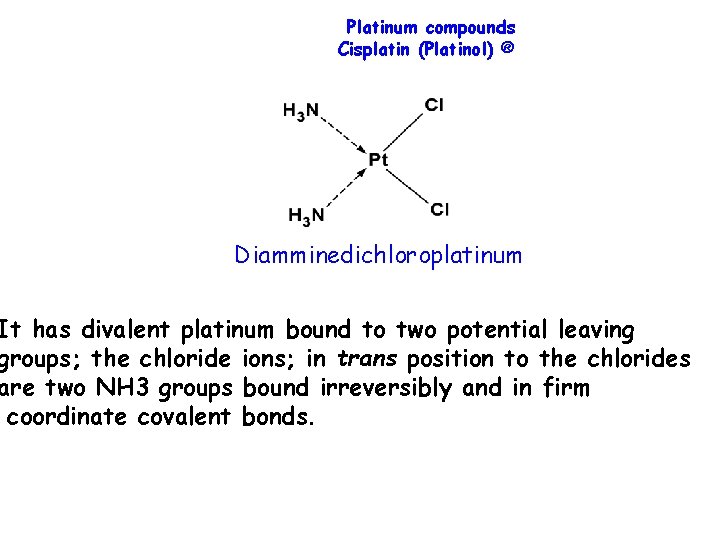
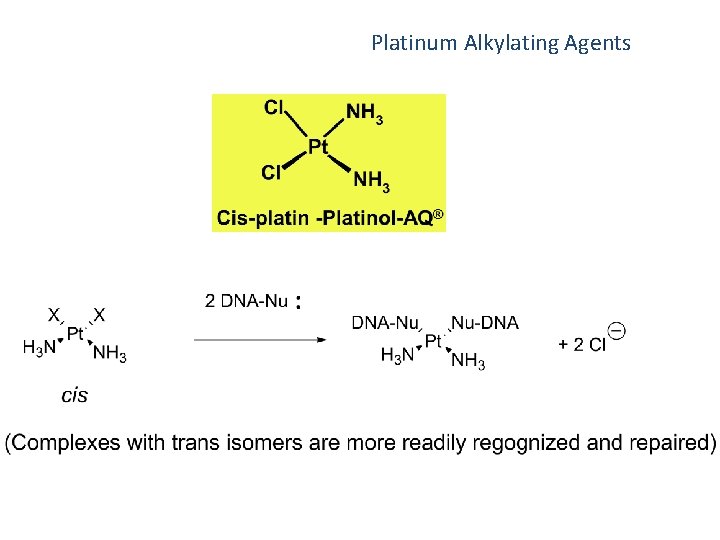
Platinum compounds Cisplatin (Platinol) ® Diamminedichloroplatinum It has divalent platinum bound to two potential leaving groups; the chloride ions; in trans position to the chlorides are two NH 3 groups bound irreversibly and in firm coordinate covalent bonds.

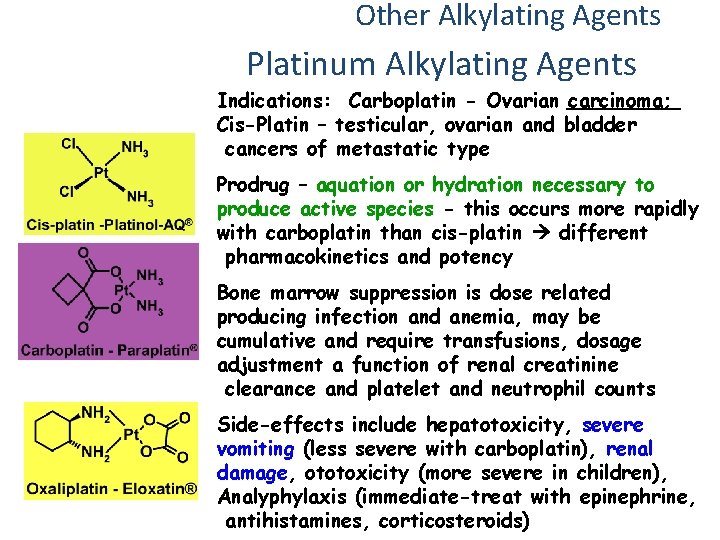
Other Alkylating Agents Platinum Alkylating Agents Indications: Carboplatin - Ovarian carcinoma; Cis-Platin – testicular, ovarian and bladder cancers of metastatic type Prodrug – aquation or hydration necessary to produce active species - this occurs more rapidly with carboplatin than cis-platin different pharmacokinetics and potency Bone marrow suppression is dose related producing infection and anemia, may be cumulative and require transfusions, dosage adjustment a function of renal creatinine clearance and platelet and neutrophil counts Side-effects include hepatotoxicity, severe vomiting (less severe with carboplatin), renal damage, ototoxicity (more severe in children), Analyphylaxis (immediate-treat with epinephrine, antihistamines, corticosteroids)

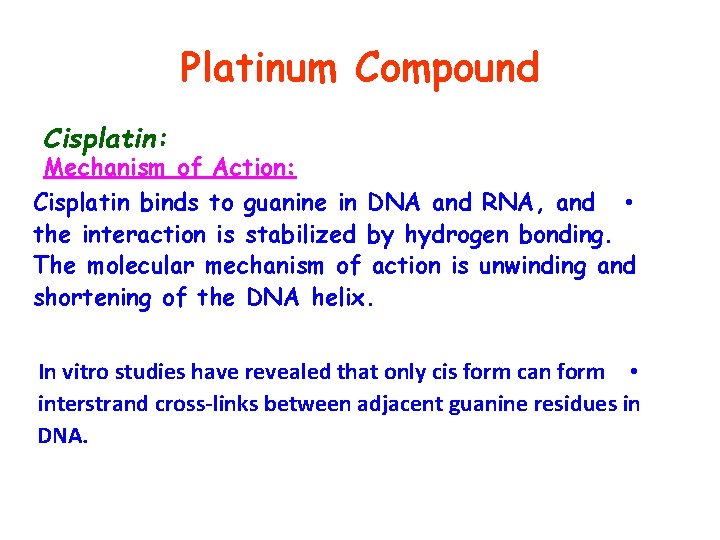
Platinum Compound Cisplatin: Mechanism of Action: Cisplatin binds to guanine in DNA and RNA, and • the interaction is stabilized by hydrogen bonding. The molecular mechanism of action is unwinding and shortening of the DNA helix. In vitro studies have revealed that only cis form can form • interstrand cross-links between adjacent guanine residues in DNA.

Platinum Alkylating Agents

Part II; Drugs acting on enzyme (Antimetabolites)

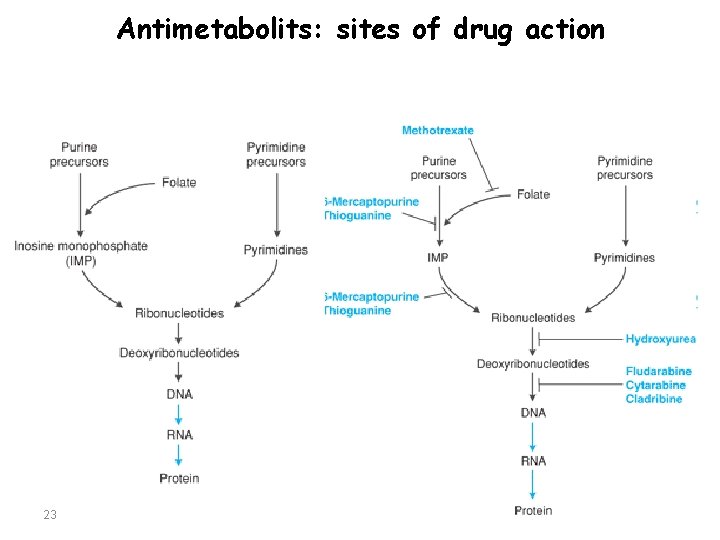
Antimetabolits: sites of drug action 23

Antimetabolites General Characteristics: Ø Antimetabolites are S phase-specific drugs that are structural analogues of essential metabolites and that interfere with DNA synthesis. Ø Myelosuppression is the dose-limiting toxicity for all drugs in this class.

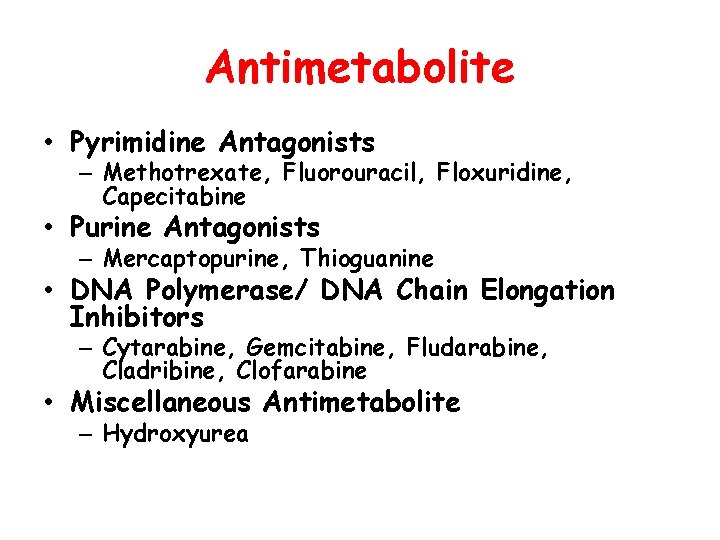
Antimetabolite • Pyrimidine Antagonists – Methotrexate, Fluorouracil, Floxuridine, Capecitabine • Purine Antagonists – Mercaptopurine, Thioguanine • DNA Polymerase/ DNA Chain Elongation Inhibitors – Cytarabine, Gemcitabine, Fludarabine, Cladribine, Clofarabine • Miscellaneous Antimetabolite – Hydroxyurea

Pyrimidine Antagonist • d. TMP Synthesis Inhibitors – Direct inhibitor: Fluorouracil, Floxuridine, Tegafur. – Indirect inhibitors: Methotrexate

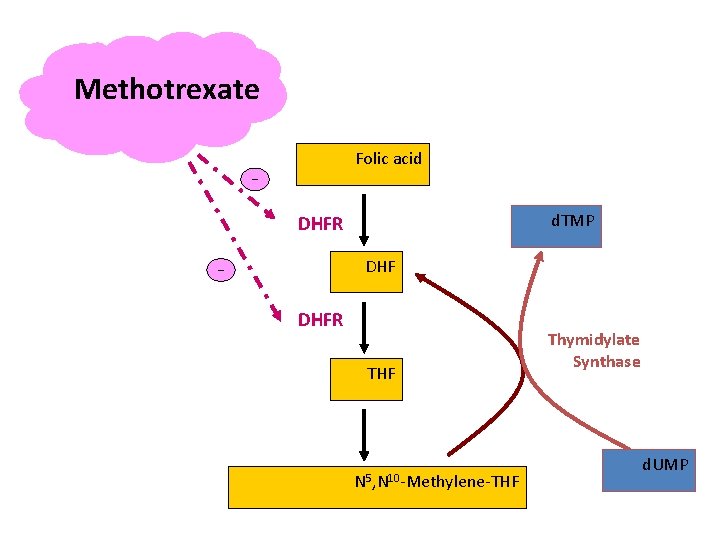
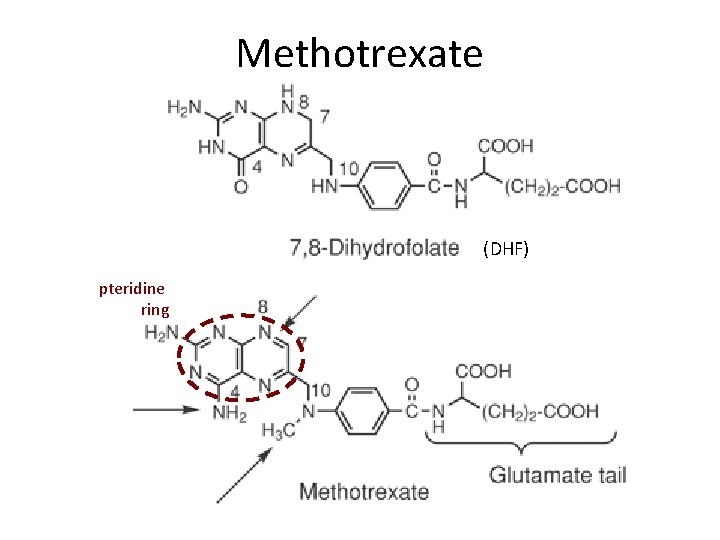
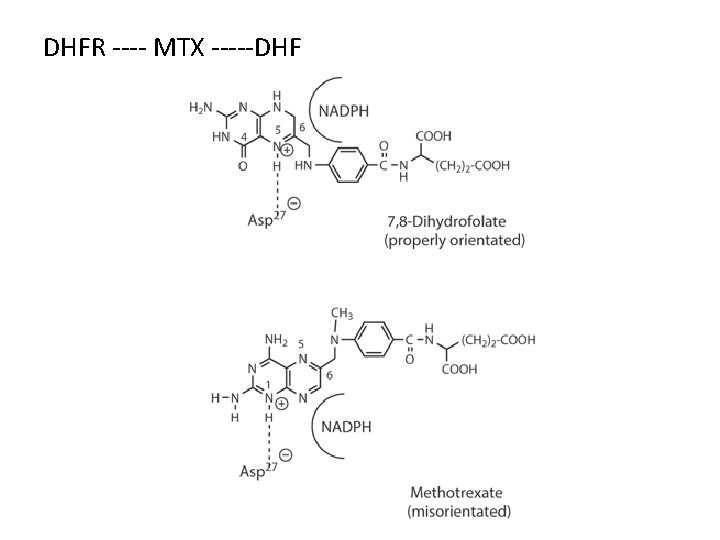
Antimetabolites—Folic Acid Antagonist Methotrexate (MTX) Mechanism of Action: Ø The structures of MTX and folic acid are similar. MTX is actively transported into mammalian cells and inhibits dihydrofolate reductase, the enzyme that normally converts dietary folate to the tetrahydrofolate form required for thymidine and purine synthesis.

Methotrexate Folic acid _ d. TMP DHFR DHF _ DHFR THF N 5, N 10 -Methylene-THF Thymidylate Synthase d. UMP
![Antimetabolites Folic Acid Antagonist Methotrxate LNp2 4 diamino6 pteridinylmethylmethylaminobenzoylglutamic acid Folic acid Antimetabolites—— Folic Acid Antagonist Methotrxate L-(+)-N-[p[[2, 4 -diamino-6 pteridinyl)methyl]methylamino]-benzoyl]glutamic acid Folic acid](https://slidetodoc.com/presentation_image_h/d11c89e8d4481d8e2a3abeb978624807/image-29.jpg)
Antimetabolites—— Folic Acid Antagonist Methotrxate L-(+)-N-[p[[2, 4 -diamino-6 pteridinyl)methyl]methylamino]-benzoyl]glutamic acid Folic acid

Methotrexate (DHF) pteridine ring

DHFR ---- MTX -----DHF

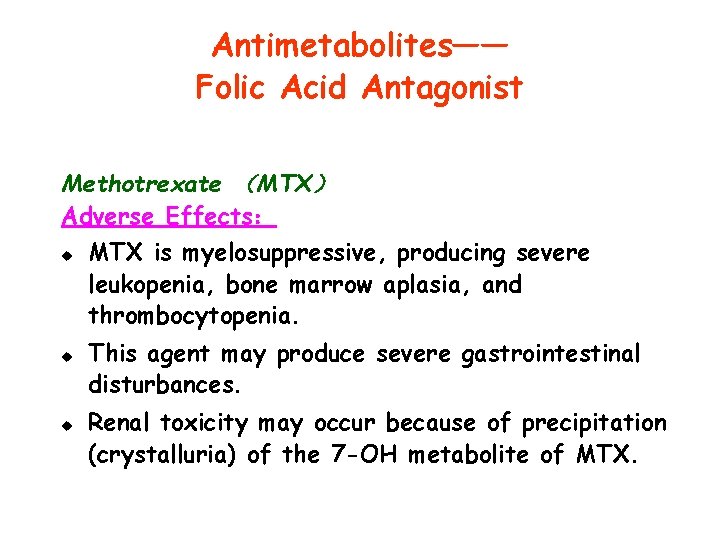
Antimetabolites—— Folic Acid Antagonist Methotrexate (MTX) Adverse Effects: u u u MTX is myelosuppressive, producing severe leukopenia, bone marrow aplasia, and thrombocytopenia. This agent may produce severe gastrointestinal disturbances. Renal toxicity may occur because of precipitation (crystalluria) of the 7 -OH metabolite of MTX.

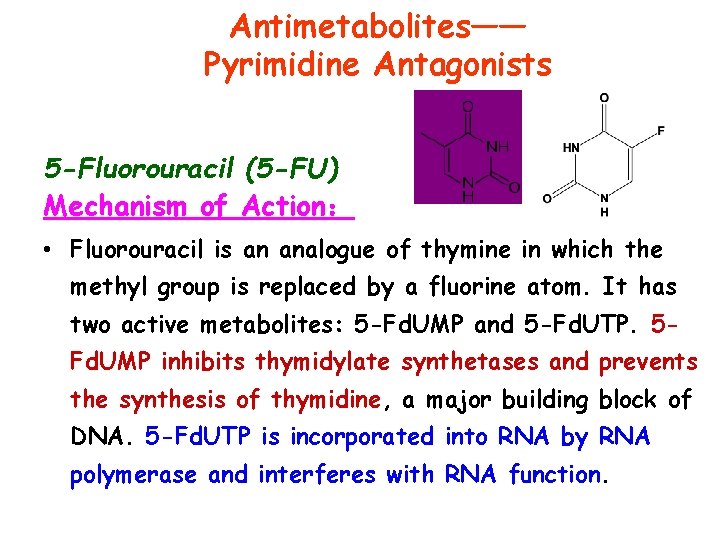
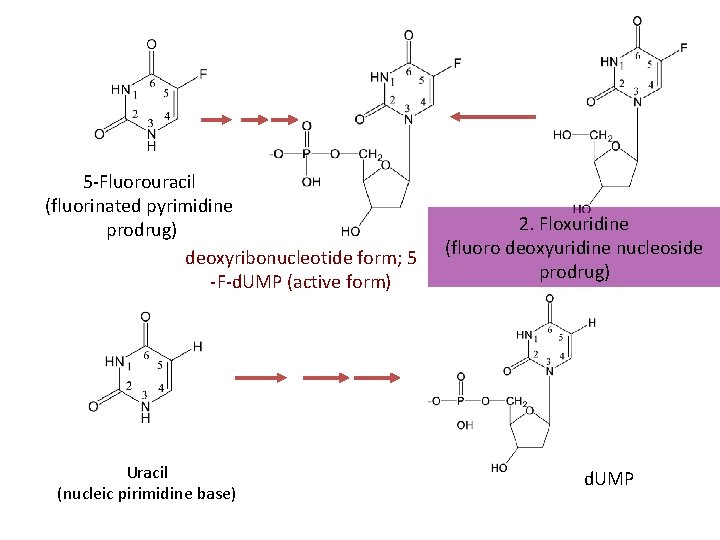
Antimetabolites—— Pyrimidine Antagonists 5 -Fluorouracil (5 -FU) Mechanism of Action: • Fluorouracil is an analogue of thymine in which the methyl group is replaced by a fluorine atom. It has two active metabolites: 5 -Fd. UMP and 5 -Fd. UTP. 5 Fd. UMP inhibits thymidylate synthetases and prevents the synthesis of thymidine, a major building block of DNA. 5 -Fd. UTP is incorporated into RNA by RNA polymerase and interferes with RNA function.

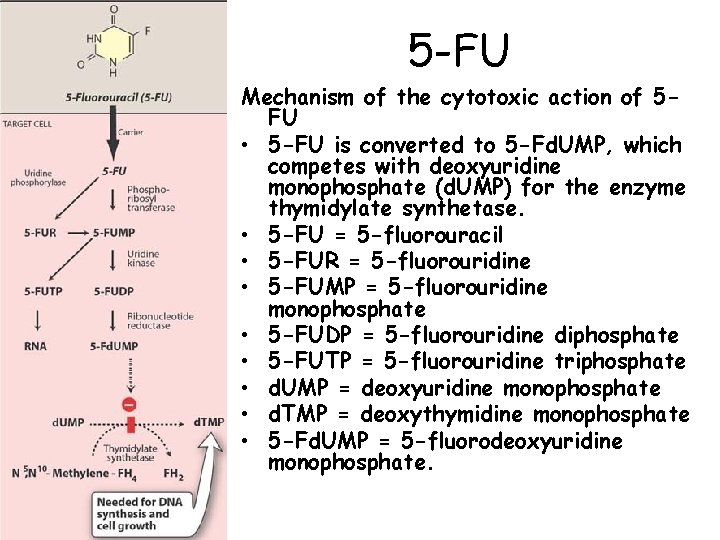
5 -FU Mechanism of the cytotoxic action of 5 FU • 5 -FU is converted to 5 -Fd. UMP, which competes with deoxyuridine monophosphate (d. UMP) for the enzyme thymidylate synthetase. • 5 -FU = 5 -fluorouracil • 5 -FUR = 5 -fluorouridine • 5 -FUMP = 5 -fluorouridine monophosphate • 5 -FUDP = 5 -fluorouridine diphosphate • 5 -FUTP = 5 -fluorouridine triphosphate • d. UMP = deoxyuridine monophosphate • d. TMP = deoxythymidine monophosphate • 5 -Fd. UMP = 5 -fluorodeoxyuridine monophosphate. 34

Antimetabolites—— Pyrimidine Antagonists 5 -Fluorouracil (5 -FU) Adverse Effects: • Fluorouracil may cause nausea and vomiting, myelosuppression, and oral and gastrointestinal ulceration. Nausea and vomitting are usually mild. • With fluorouracil, myelosuppression is more problematic after bolus injections, whereas mucosal damage is dose-limiting with continuous infusions.

5 -Fluorouracil (fluorinated pyrimidine prodrug) deoxyribonucleotide form; 5 -F-d. UMP (active form) Uracil (nucleic pirimidine base) 2. Floxuridine (fluoro deoxyuridine nucleoside prodrug) d. UMP

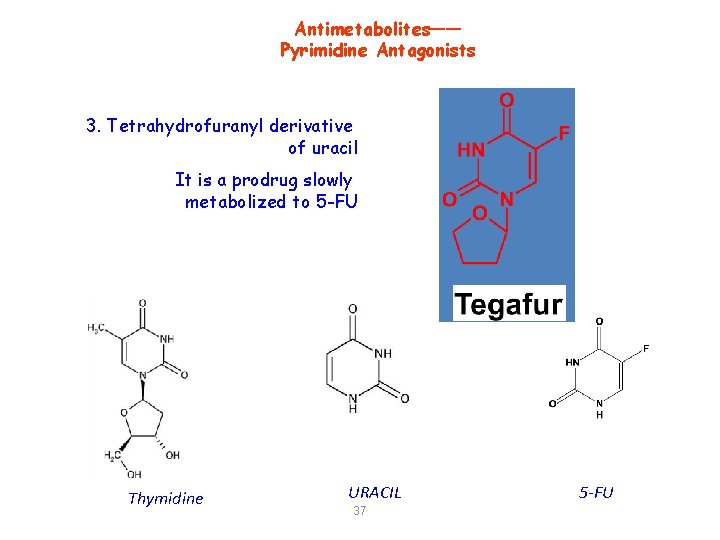
Antimetabolites—— Pyrimidine Antagonists 3. Tetrahydrofuranyl derivative of uracil It is a prodrug slowly metabolized to 5 -FU Thymidine URACIL 37 5 -FU

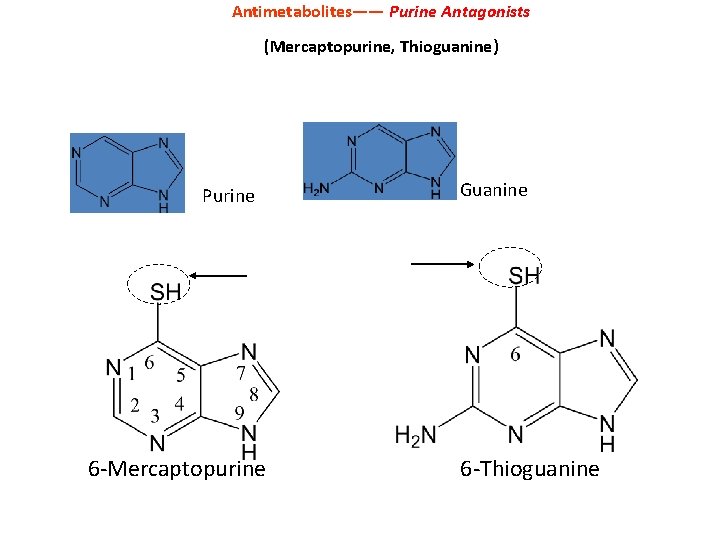
Antimetabolites—— Purine Antagonists (Mercaptopurine, Thioguanine) Purine 6 -Mercaptopurine Guanine 6 -Thioguanine

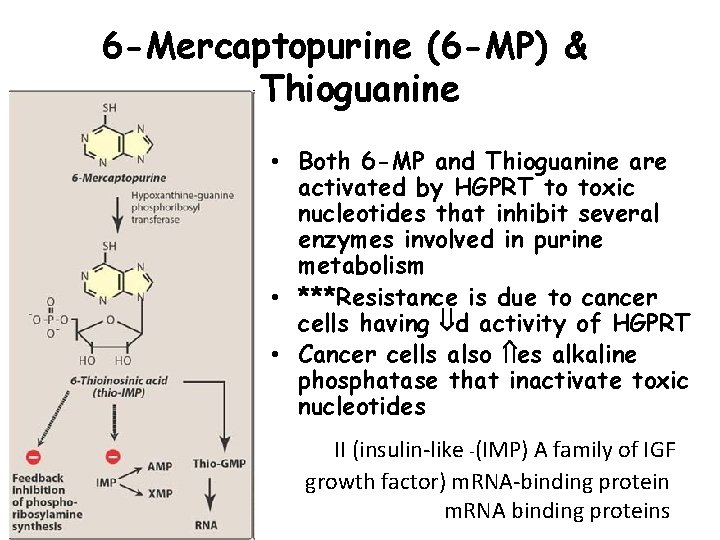
6 -Mercaptopurine (6 -MP) & Thioguanine • Both 6 -MP and Thioguanine are activated by HGPRT to toxic nucleotides that inhibit several enzymes involved in purine metabolism • ***Resistance is due to cancer cells having d activity of HGPRT • Cancer cells also es alkaline phosphatase that inactivate toxic nucleotides 39 II (insulin-like -(IMP) A family of IGF growth factor) m. RNA-binding protein m. RNA binding proteins

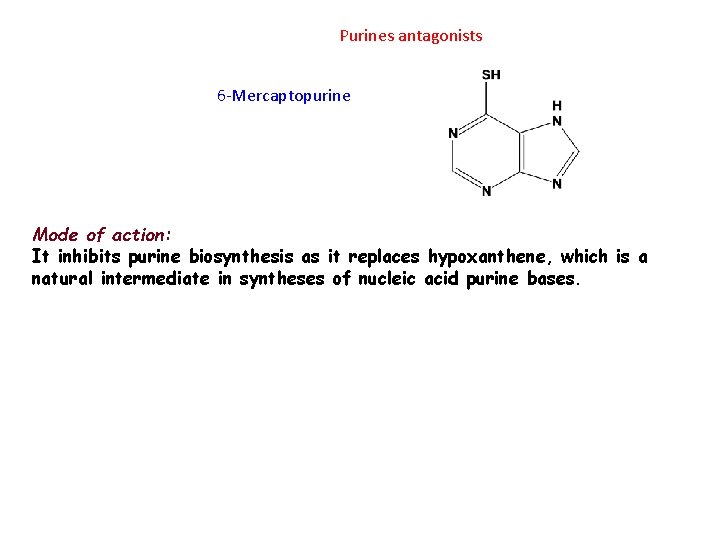
Purines antagonists 6 -Mercaptopurine Mode of action: It inhibits purine biosynthesis as it replaces hypoxanthene, which is a natural intermediate in syntheses of nucleic acid purine bases.

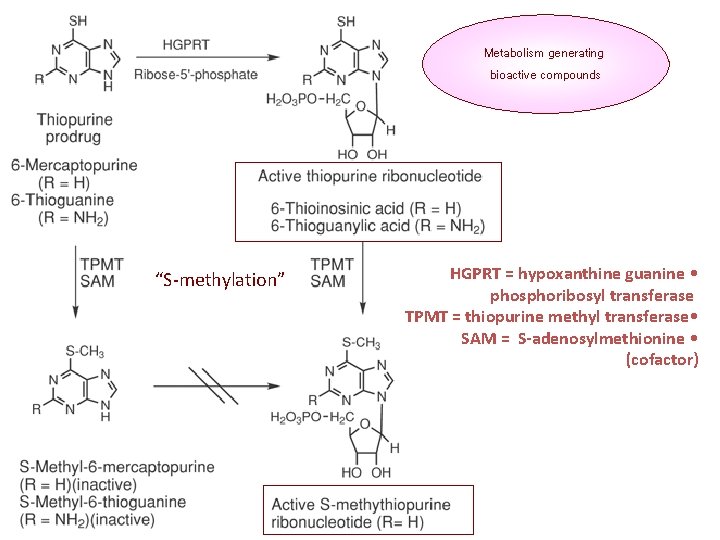
Metabolism generating bioactive compounds “S-methylation” HGPRT = hypoxanthine guanine • phosphoribosyl transferase TPMT = thiopurine methyl transferase • SAM = S-adenosylmethionine • (cofactor)

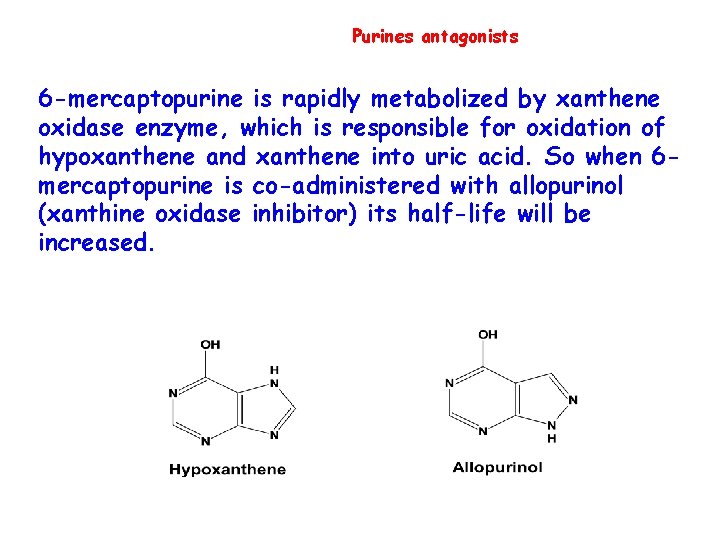
Purines antagonists 6 -mercaptopurine is rapidly metabolized by xanthene oxidase enzyme, which is responsible for oxidation of hypoxanthene and xanthene into uric acid. So when 6 mercaptopurine is co-administered with allopurinol (xanthine oxidase inhibitor) its half-life will be increased.

6 -MP & Allopurinol • 6 -MP is metabolized in the liver by xanthine oxidase and the inactive metabolites are excreted in the urine • ***Allopurinol is used frequently to treat/prevent hyperuricemia caused by many anticancer drugs. • If Allopurinol is used with 6 -MP then the dose of 6 -MP is reduced by more than 75% 43


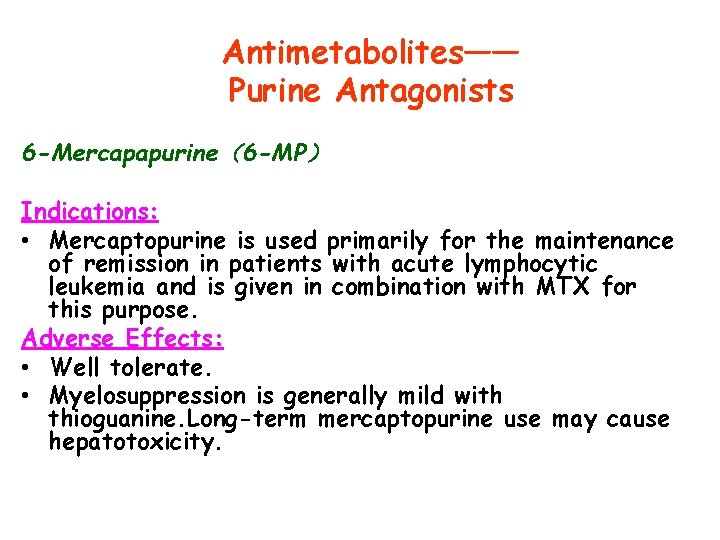
Antimetabolites—— Purine Antagonists 6 -Mercapapurine(6 -MP) Indications: • Mercaptopurine is used primarily for the maintenance of remission in patients with acute lymphocytic leukemia and is given in combination with MTX for this purpose. Adverse Effects: • Well tolerate. • Myelosuppression is generally mild with thioguanine. Long-term mercaptopurine use may cause hepatotoxicity.

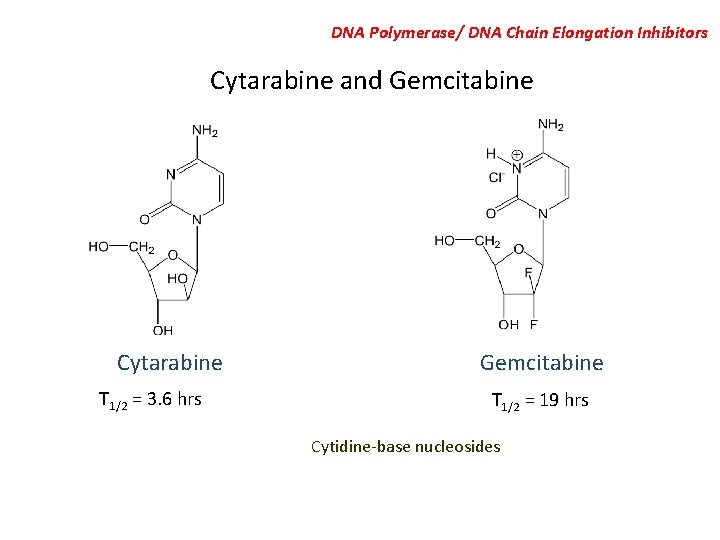
DNA Polymerase/ DNA Chain Elongation Inhibitors Cytarabine and Gemcitabine Cytarabine T 1/2 = 3. 6 hrs Gemcitabine T 1/2 = 19 hrs Cytidine-base nucleosides

DNA Polymerase/ DNA Chain Elongation Inhibitors Cytarabine Indications: • Cytarabine has a narrow clinical spectrum and is primarily used in combination with daunorubicin or thioguanine for the treatment of acute nonlymphocytic leukemia. Adverse Effects: • High doses of cytarabine can damage the liver, heart, and other organs.

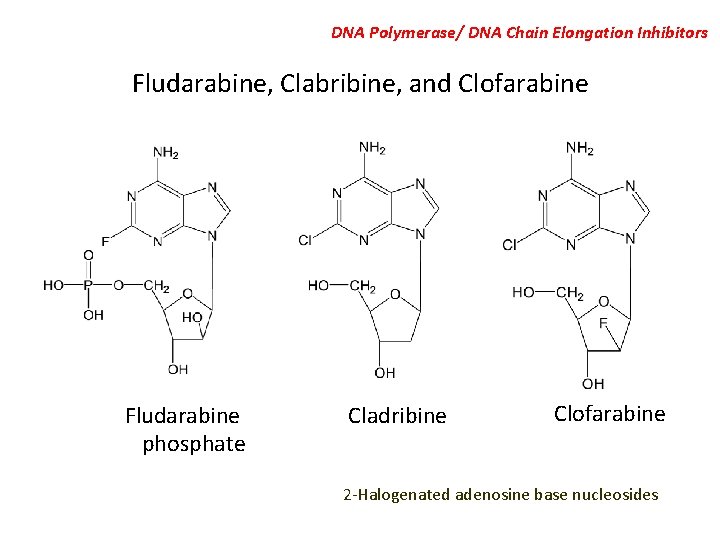
DNA Polymerase/ DNA Chain Elongation Inhibitors Fludarabine, Clabribine, and Clofarabine Fludarabine phosphate Cladribine Clofarabine 2 -Halogenated adenosine base nucleosides

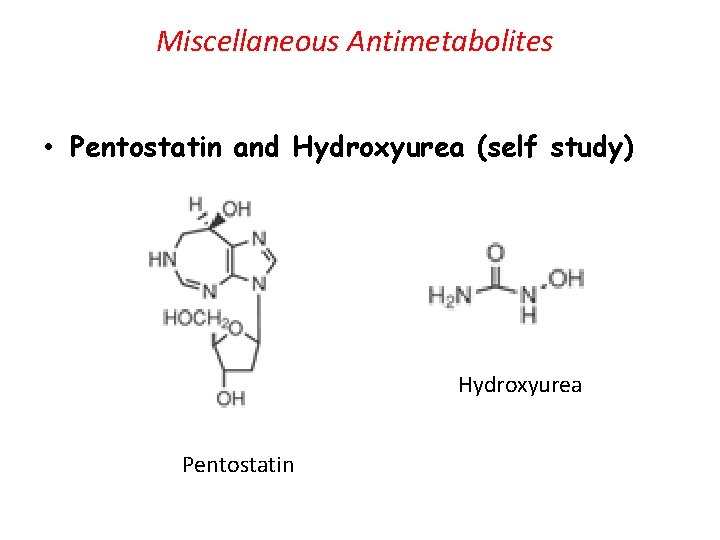
Miscellaneous Antimetabolites • Pentostatin and Hydroxyurea (self study) Hydroxyurea Pentostatin

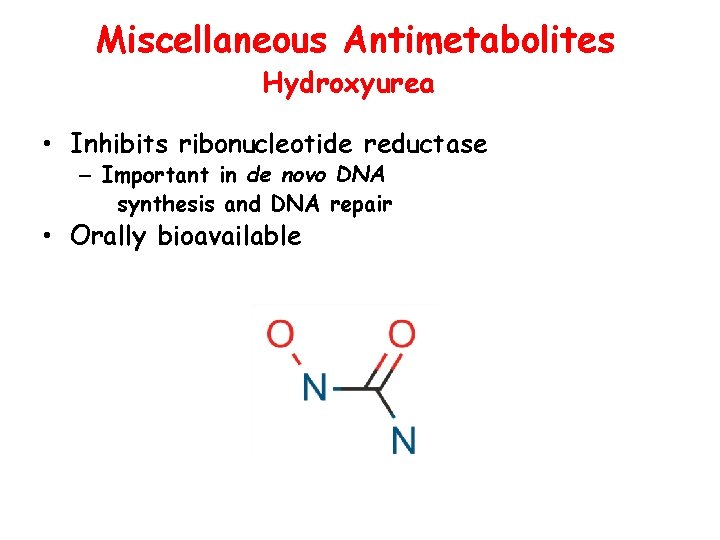
Miscellaneous Antimetabolites Hydroxyurea • Inhibits ribonucleotide reductase – Important in de novo DNA synthesis and DNA repair • Orally bioavailable