Antibiotic sensitivity test Many bacteria have developed the
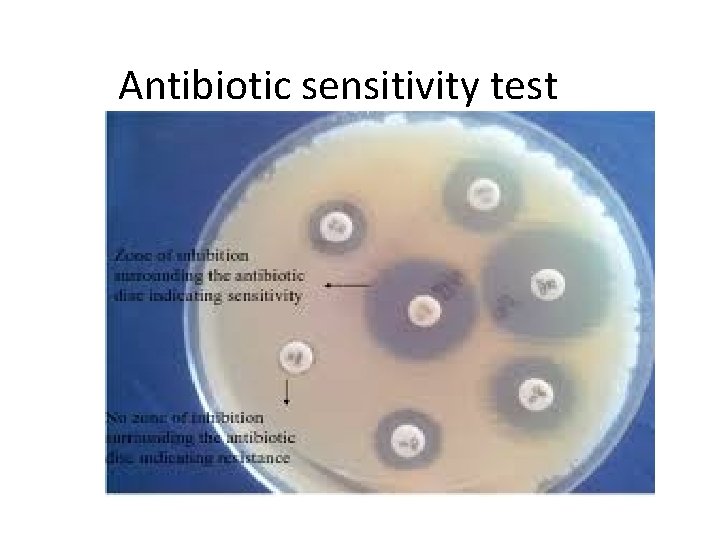
Antibiotic sensitivity test

– Many bacteria have developed the ability to become resistant to antibiotics. – These bacteria are now a major threat in our hospitals. – Antibiotic resistant bacteria include Methicillin Resistant Staphylococcus aureus (MRSA) •

Materials Required • • Petriplate containing microbial culture(For . 1 example, Escherichia coli) Inoculation loop. 2 Bunsen burner. 3 Saline solution. 4 Mc. Farland solution. 5 MHA plate. 6 Cotton swab. 7 Antibiotic disks(Streptomycin (S), Ciprofloxacin . 8 (CIP), Chloramphenicol (C), Doxycycline (D), Penicillin G (P), Gentamycin (G) Tooth pick. 9 Incubator. 10

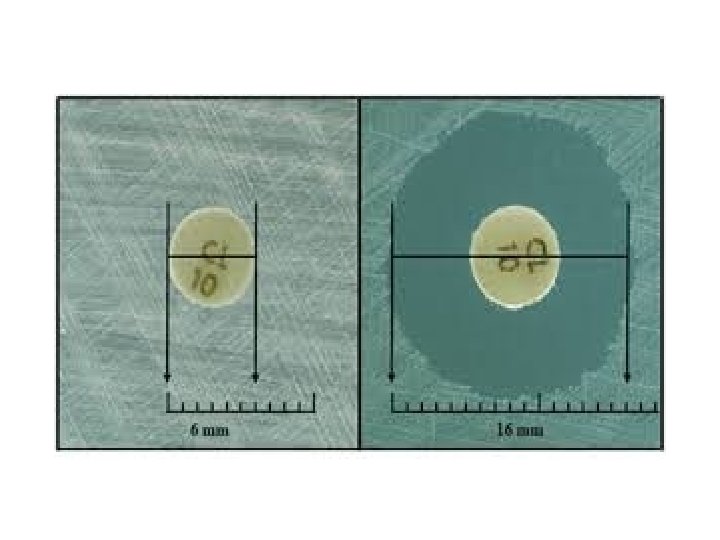
e • • Select a pure culture plate of one of the organisms to be tested. • Aseptically emulsify a colony from the plate in the sterile saline solution. Mix it • thoroughly to ensure that no solid material from the colony is visible in the saline solution. Repeat until the turbidity of the saline solution visually match that of the • standard turbidity. Take a sterile swab and dip it into the broth culture of organism. • Gently squeeze the swab against the inside of the tube in order to remove excess • fluid in the swab. Take a sterile Mueller-Hinton agar (MHA) plate or a nutrient agar (NA) plate. • Use the swab with the test organism to streak a MHA plate or a NA plate for a • lawn of growth. After the streaking is complete, allow the plate to dry for 5 minutes. • Antibiotic discs can be placed on the surface of the agar using sterilized forceps. • Gently press the discs onto the surface of the agar using flame sterilized forceps • or inoculation loop. Carefully invert the inoculated plates and incubate for 24 hours at 37° C. • After incubation, use a metric ruler to measure the diameter of the zone of • inhibition for each antibiotic used. Compare the measurement obtained from the individual antibiotics with the • standard table to determine the sensitivity zone. Compare the measurement obtained from the individual antibiotics to the • standard table to determine whether the tested bacterial species is sensitive or resistant to the tested antibiotic.

* Müller-Hinton agar is a microbiological growth medium that is commonly used for antibiotic sensetivity testing. It is also used to isolate and maintain Neisseria and Moraxella species. It typically contains: 1 -2. 0 g beef extract 2 -17. 5 g casein hydrolysate 3 -1. 5 g starch 4 -17. 0 g agar dissolved in 1 liter of distilled water. 5 - p. H adjusted to neutral at 25 °C.

It has a few properties that make it excellent for antibiotic use. 1 - First of all, it is a non-selective, nondifferential medium. This means that almost all organisms plated on here will grow. 2 -Additionally, it contains starch. Starch is known to absorb toxins released from bacteria, so that they cannot interfere with the antibiotics. 3 -it is a loose agar. This allows for better diffusion of the antibiotics than most other plates, a better diffusion leads to a truer zone of inhibition.
- Slides: 8